No products in the cart.
Sale
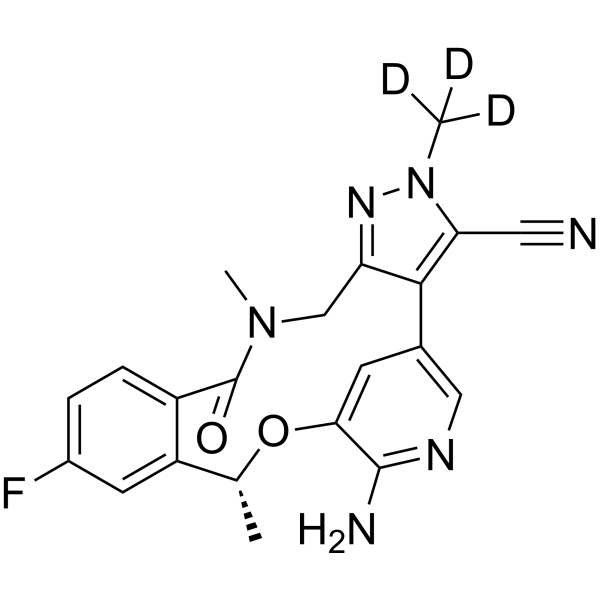
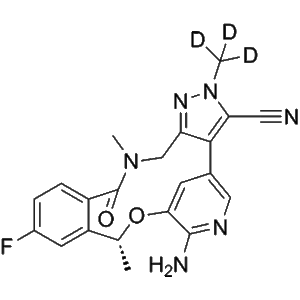
Deulorlatinib | CAS No. 2131126-33-3
Original price was: $42.00.$36.00Current price is: $36.00.
Deulorlatinib (Compound A) is a deuterium-stabilized analogue of Lorlatinib, designed to improve pharmacokinetics and metabolic stability. It selectively inhibits ALK kinase activity and is widely used in research related to ALK-positive advanced NSCLC.
Description
Contents
hide
Product Description
Deulorlatinib (Compound A) is a next-generation deuterated tyrosine kinase inhibitor (TKI) that builds upon the design and therapeutic potential of Lorlatinib (PF-06463922). Through the incorporation of deuterium atoms at specific metabolic “soft spots”, Deulorlatinib exhibits improved pharmacokinetic stability, reduced metabolic clearance, and potentially enhanced bioavailability, making it an optimized research analogue for the study of ALK-driven malignancies.
Research Background
ALK (anaplastic lymphoma kinase) rearrangements are among the most significant oncogenic drivers identified in subsets of non-small cell lung cancer (NSCLC), an aggressive disease associated with poor outcomes if untreated. The discovery of ALK inhibitors, such as crizotinib, revolutionized treatment paradigms, but resistance inevitably arises, frequently involving secondary ALK kinase domain mutations (e.g., L1196M, G1202R, G1269A).
Lorlatinib was developed as a third-generation ALK inhibitor to address resistance, but metabolic degradation and dose-limiting toxicities remain areas of concern. Deulorlatinib, as a deuterated derivative, retains the core inhibitory profile of Lorlatinib but offers enhanced pharmacological properties, thereby serving as an important experimental tool for improving our understanding of ALK-targeted therapy optimization.
Key Features
Deuterium Substitution: Enhances metabolic stability by reducing oxidative metabolism.
Potent ALK Inhibition: Retains strong ATP-competitive inhibitory activity against wild-type ALK and resistant mutants.
Resistance Mutation Coverage: Designed to address difficult-to-treat mutations such as G1202R, a common mechanism of Lorlatinib resistance.
Improved Pharmacokinetics: Deuteration slows clearance, prolonging systemic exposure in preclinical models.
Potential for CNS Activity: Similar to Lorlatinib, designed for blood-brain barrier penetration, making it suitable for studying brain metastasis in ALK-positive NSCLC.
Research Applications
NSCLC Oncology Research: Investigating ALK fusion-driven lung cancers and resistance pathways.
Drug Resistance Studies: Understanding how ALK mutations alter inhibitor binding and identifying solutions.
Deuterated Drug Development: Serves as a case study in deuteration technology for improving small molecule inhibitors.
Brain Metastasis Models: Potential to explore treatment efficacy in CNS disease models.
Structure-Activity Relationship Studies: Comparison with Lorlatinib highlights how subtle structural modifications alter drug metabolism.
Deulorlatinib thus represents a critical tool compound for oncological researchers, bridging the gap between Lorlatinib’s established activity and future-generation ALK inhibitors with improved therapeutic indices.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Deulorlatinib |
| CAS Number | 2131126-33-3 |
| Synonyms | Compound A, Deuterated Lorlatinib analogue |
| Parent Molecule | Lorlatinib (PF-06463922) |
| Chemical Class | Deuterated small molecule TKI |
| Molecular Target | ALK kinase (wild type and mutants) |
| Mechanism | ATP-competitive kinase inhibition |
| Resistance Coverage | Effective against L1196M, G1269A, and G1202R mutations |
| Biological Effects | Blocks ALK phosphorylation and downstream oncogenic signaling |
| Applications | NSCLC research, resistance pathway analysis, CNS metastasis studies |
| Appearance | White to off-white crystalline powder |
| Purity | ≥98% (HPLC) |
| Solubility | DMSO, ethanol |
| Storage Conditions | -20°C, light-protected, sealed |
| Shelf Life | ≥24 months |
| QC Tests | HPLC, NMR, Mass spectrometry |
Mechanism of Action
Deulorlatinib inhibits ALK tyrosine kinase activity by binding competitively to the ATP-binding pocket of the enzyme, thereby blocking phosphorylation and activation of downstream signaling cascades that drive cancer cell proliferation and survival.
1. ATP-Competitive Inhibition
Directly binds ALK kinase domain.
Prevents ATP-mediated phosphorylation events.
Halts downstream activation of PI3K/AKT, MAPK, and JAK/STAT pathways.
2. Mutation Resistance Profile
Like Lorlatinib, Deulorlatinib is effective against several resistance mutations:
L1196M (Gatekeeper mutation): Often confers resistance to crizotinib.
G1269A: Associated with second-generation TKI resistance.
G1202R: Considered the most difficult-to-target ALK mutation; Deulorlatinib shows inhibitory activity here as well.
3. Role of Deuterium Substitution
Substituting hydrogen atoms with deuterium reduces oxidative metabolism at vulnerable sites.
Leads to slower drug clearance, reduced toxic metabolite formation, and longer duration of action.
Provides a research model for the impact of isotope substitution on pharmacology.
4. CNS Penetration
Deulorlatinib, like Lorlatinib, was designed to cross the blood-brain barrier. This is crucial because up to 50% of ALK-positive NSCLC patients develop brain metastases during disease progression.
5. Research Impact
Enhances understanding of ALK resistance evolution.
Serves as a comparative benchmark against Lorlatinib in preclinical models.
Demonstrates how structural optimization improves drug-like properties without sacrificing potency.

Side Effects
Deulorlatinib is for research use only. Experimental data and extrapolation from Lorlatinib studies suggest the following potential effects:
Metabolic Effects: Altered lipid metabolism, possibly leading to hyperlipidemia in preclinical models.
Neurological Effects: Due to CNS penetration, may influence neuronal activity, cognition, or behavior in animal studies.
Cardiac Considerations: Potential QT interval changes in electrophysiological studies.
Hepatic Effects: Possible elevation of liver enzyme levels in long-term administration.
Resistance Induction: Chronic exposure may select for secondary ALK mutations, useful for resistance modeling.
Laboratory Handling Precautions
Research use only.
Use gloves, goggles, and protective equipment.
Follow BSL-2 safety protocols.
Disclaimer
This compound is intended for laboratory research only. It is not approved for clinical or veterinary applications.
Keywords
Deulorlatinib, Compound A, deuterated Lorlatinib, ALK inhibitor, NSCLC research, kinase inhibitor, resistance mutation inhibitor, anticancer small molecule, CAS 2131126-33-3.Peptide Manufacturer-Wholesaler
Shipping Guarantee
All shipments include tax-inclusive clearance and are fully insured against loss or damage.
Transaction Guarantee
Payments accepted via T/T, PayPal, cryptocurrency, and additional secure methods upon request.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 56 × 28 × 56 cm |
1. What is Deulorlatinib?
A deuterated derivative of Lorlatinib, optimized for improved pharmacokinetic stability.
2. What is its CAS number?
CAS No. 2131126-33-3.
3. How does Deulorlatinib differ from Lorlatinib?
It incorporates deuterium atoms, reducing metabolism and extending duration of action.
4. What is its primary target?
Anaplastic lymphoma kinase (ALK), including wild-type and resistant mutants.
5. Which resistance mutations can it inhibit?
L1196M, G1269A, and G1202R.
6. Does Deulorlatinib penetrate the CNS?
Yes, like Lorlatinib, it is designed for blood-brain barrier permeability.
7. What is its research application?
Primarily for ALK-positive NSCLC, drug resistance studies, and deuteration research.
8. What are its side effects?
Potential CNS, hepatic, metabolic, and cardiac effects in preclinical models.
9. Is it approved for human use?
No, it is strictly for research use only.
10. What are its storage conditions?
Store at -20°C, protected from light and moisture.
11. What payment methods are accepted?
T/T, PayPal, cryptocurrency, and other secure transactions.
12. How is it shipped?
Shipped with customs clearance, insurance, and damage/loss protection.











Reviews
There are no reviews yet.