No products in the cart.
Fosfomycin Sodium Injection 250?ml:6?g – Wholesale & Retail (Lab?Grade)
$1.00
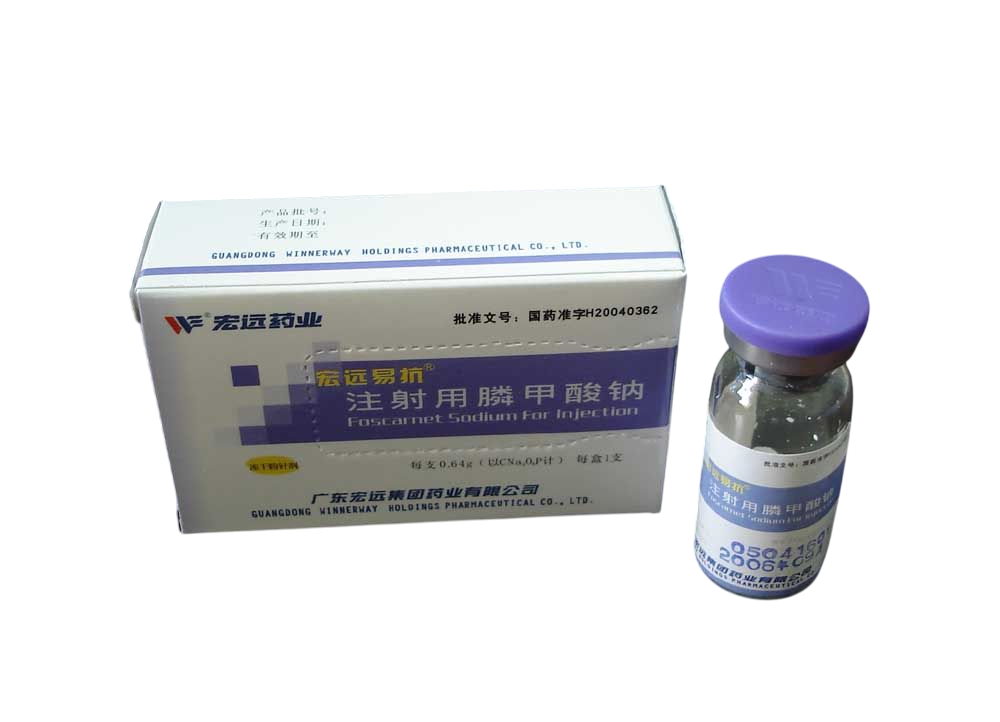
Fosfomycin Sodium Injection (250?ml:6?g vial) is a sterile injectable antibiotic derived from phosphonic acid derivatives, manufactured by Guangdong Hongyuan Group Pharmaceutical Co., Ltd., approved under H20040362. This lab-grade product supports wholesale and retail distribution for research applications only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
Fosfomycin Sodium Injection 250?ml:6?g is a high-purity sterile antibiotic solution, formulated for intravenous administration. As a phosphonic acid derivative, it exerts bactericidal activity by inactivating enolpyruvyl transferase, disrupting early-stage bacterial cell wall synthesis. This pharmaco-grade formulation, produced by Guangdong Hongyuan Group Pharmaceutical Co., Ltd., is approved under Chinese licence H20040362. Each vial contains 6?g sodium fosfomycin in 250?ml aqueous solution. Available for both wholesale and retail sales. Intended for laboratory research purposes only.
Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Fosfomycin Sodium Injection |
| Volume & Strength | 250?ml containing 6?g sodium fosfomycin |
| Dosage Form | Injectable solution |
| Packaging Unit | 1 vial per bottle |
| Manufacturer | Guangdong Hongyuan Group Pharmaceutical Co., Ltd. |
| Approval Number | ???? H20040362 |
| Drug Standard Code | 86900289000442 |
| Barcode | Not yet assigned—contact support |
| CAS Number | 63?07?0 (Fosfomycin sodium) |
| Molecular Formula | C3H7NaO4P (approximate standard) |
Mechanism of Action & Research Applications
Fosfomycin Sodium inhibits bacterial cell wall synthesis by targeting the enzyme enolpyruvyl transferase (MurA), preventing the condensation of UDP?N?acetylglucosamine with phosphoenolpyruvate. This mechanism yields potent bactericidal activity, particularly against multidrug-resistant Gram?negative pathogens like Carbapenem?resistant Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii. In research, Fosfomycin Sodium Injection 250?ml:6?g is widely used in experimental models of systemic infection, pneumonia, intra?abdominal sepsis, urinary tract infection, bone and joint infections, and endocarditis. Use of this product aids scientific exploration of antimicrobial resistance and new combination therapies. Intended solely for laboratory research use.
Side Effects (For Reference Only in Models)
In experimental or clinical-like models, observed effects may include gastrointestinal symptoms (nausea, diarrhea), headache, dizziness, rash, and potential renal or hematological changes. Cardiac rhythm disturbances and electrolyte imbalance may occur in high doses or prolonged exposure models. These findings serve as reference for model design and dosing safety considerations.
Disclaimer
Fosfomycin Sodium Injection 250?ml:6?g is strictly intended for laboratory research use only. Not for human or veterinary therapeutic use.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |










Reviews
There are no reviews yet.