Gefitinib Tablets 0.25?g Price comparison
$1.00
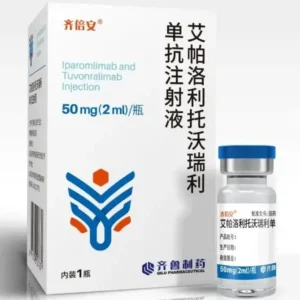
Gefitinib Tablets (brand name: Tifini / Diy) are small-molecule EGFR tyrosine kinase inhibitors designed for use in experimental cancer biology, especially in studies related to non-small cell lung cancer (NSCLC) with EGFR mutations. Each box contains 10 tablets, each with 0.25 grams (250 mg) of gefitinib. Manufactured by Jiangsu Tasly Diy Pharmaceutical Co., Ltd., this product is registered under Chinese NMPA approval H20203704.
?? Strictly for laboratory research use.?Please consult the staff for other purposes?
描述
Product Specifications
-
Product Name: Gefitinib Tablets (Tifini / Diy)
-
Generic Name: Gefitinib
-
Formulation: Oral film-coated tablet
-
Strength: 250 mg (0.25 g) per tablet
-
Pack Size: 10 tablets per box
-
Dosage Form: Tablet
-
Approval Number: H20203704 (NMPA, China)
-
Product Code: 86901500001279
-
Manufacturer: Jiangsu Tasly Diy Pharmaceutical Co., Ltd.
-
Barcode: 6938518100001
-
CAS Number: 184475-35-2
-
Storage: Store below 30?°C in a dry place
-
Intended Use: For laboratory research only
Mechanism of Action
Gefitinib is a selective tyrosine kinase inhibitor that blocks the activity of the epidermal growth factor receptor (EGFR), particularly in cancer cells with activating EGFR mutations. It inhibits downstream signaling pathways such as PI3K/Akt and MAPK/ERK, thereby suppressing tumor cell proliferation and promoting apoptosis.
Research Applications & Pharmacology
-
EGFR-mutant NSCLC models
-
Tumor biology, growth inhibition, and apoptosis studies
-
Resistance pathway research (e.g. T790M, MET amplification)
-
Drug combination experiments (e.g. osimertinib, chemotherapy)
-
In vitro: cell viability, signaling studies
-
In vivo: xenograft tumor inhibition, pharmacokinetics
-
Metabolism: Mainly via CYP3A4; excreted in feces
-
Half-life: 6–49 hours depending on species and dosage
Safety Profile & Side Effects
Common Observed Effects in Preclinical Studies:
-
Gastrointestinal: diarrhea, nausea, vomiting
-
Skin: rash, dryness
-
Mild liver enzyme elevation
-
Rare hematologic suppression (anemia, neutropenia)
Important Handling Notes:
-
Use proper lab PPE (gloves, mask, lab coat)
-
Avoid skin or eye contact
-
Do not inhale tablet powder
-
Store away from humidity
-
Dispose of residues per hazardous waste protocol
?? Research-Use Disclaimer
This product is intended exclusively for laboratory research use. It is not approved for clinical, therapeutic, diagnostic, or veterinary purposes. Improper handling or unauthorized application may pose serious health risks.
其他信息
| 重量 | 1.1 公斤 |
|---|---|
| 尺寸 | 18 × 16 × 18 厘米 |








评价
目前还没有评价