No products in the cart.
Sale
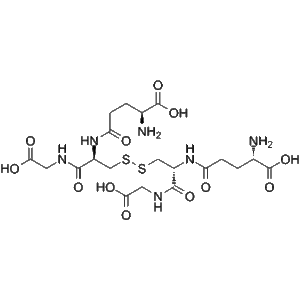
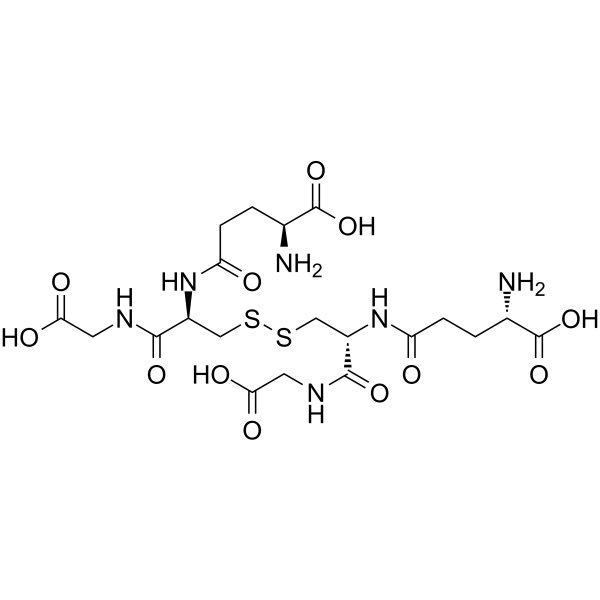
Glutathione Oxidized | 5mg and 10mg high-purity peptides
Original price was: $65.00.$53.00Current price is: $53.00.
High-purity Glutathione Oxidized (CAS 27025-41-8) is a research-grade lyophilized peptide suitable for laboratory studies on redox biology, oxidative stress regulation, and glutathione-dependent enzymatic pathways. Factory-manufactured and validated for analytical and mechanistic investigations.(For research use only. Not for human or veterinary use.)
Description
Contents
hide
Product Description
Glutathione Oxidized (CAS 27025-41-8), commonly referred to as GSSG, is a high-purity lyophilized peptide widely utilized in laboratory research to investigate oxidative stress, redox biology, and glutathione-dependent enzymatic processes. As the oxidized form of glutathione, it plays a pivotal role in the study of thiol-disulfide homeostasis, redox potential regulation, and cellular defense mechanisms against reactive oxygen species (ROS). Factory-manufactured under GMP-aligned protocols, Glutathione Oxidized offers consistent molecular integrity, batch reproducibility, and analytical quality suitable for high-precision laboratory applications.
This research-grade peptide enables detailed exploration of biochemical pathways where glutathione acts as a key redox buffer. Glutathione Oxidized serves as a substrate in enzymatic assays involving glutathione reductase, thiol-disulfide exchange reactions, and redox-dependent modulation of protein activity. Researchers leverage its predictable chemical and structural properties to probe the balance between reduced (GSH) and oxidized glutathione in controlled experimental systems, allowing for mechanistic insights into oxidative stress responses, redox signaling, and cellular antioxidant capacity.
The lyophilized form of Glutathione Oxidized ensures long-term stability and ease of integration into aqueous solutions, buffers, and experimental workflows. It dissolves readily under mild conditions, facilitating its use in spectroscopic, chromatographic, and fluorescence-based assays. Laboratories frequently utilize this peptide in high-throughput screening, multi-omic analyses, and enzyme kinetics studies to explore the role of glutathione in cellular and molecular regulatory networks.
Beyond biochemical assays, Glutathione Oxidized is instrumental in comparative studies with reduced glutathione (GSH) to elucidate redox-dependent effects on signaling cascades, protein S-glutathionylation, and metabolic regulation. Its high purity ensures reproducibility across complex experimental designs, including cell-free enzymatic models, tissue extracts, and advanced computational simulations.
Due to its well-characterized structure and predictable behavior, Glutathione Oxidized is a reliable standard for mechanistic studies of oxidative biology. Researchers employ it to validate experimental systems, benchmark enzyme activity assays, and calibrate redox measurements. The peptide’s integration into multi-step workflows and multi-omic platforms enhances the robustness of data, supporting comprehensive exploration of redox regulation, stress responses, and peptide-mediated cellular modulation.
In summary, Glutathione Oxidized (CAS 27025-41-8) is a research-grade, high-purity lyophilized peptide essential for laboratory investigations into oxidative stress, redox biology, and glutathione-dependent biochemical processes. Its consistent quality, structural integrity, and compatibility with diverse assay systems make it a foundational reagent for experimental research in cellular signaling, enzymology, and multi-omic studies.

Product Specifications
| Parameter | Detailed Specification |
|---|---|
| Product Name | Glutathione Oxidized |
| CAS Number | 27025-41-8 |
| Chemical Formula | C20H32N6O12S2 |
| Molecular Weight | 612.63 g/mol |
| Form | Lyophilized Powder |
| Purity | ≥98% (HPLC) |
| Appearance | White to off-white crystalline powder |
| Solubility | Soluble in water and aqueous buffers; solubility profile validated under mild laboratory conditions |
| Storage Conditions | −20 °C in tightly sealed, light-protected containers; avoid repeated freeze–thaw cycles |
| Packaging Options | 1 mg, 5 mg, 10 mg vials; bulk and OEM options available for research laboratories |
| Documentation Provided | Certificate of Analysis (COA), MSDS, batch QC report, HPLC chromatogram, structural verification notes |
| Manufacturing Standard | GMP-aligned, factory-controlled production with validated purification and quality control steps |
Notes
High-Purity Assurance:
Every batch of Glutathione Oxidized is synthesized and purified under strict GMP-aligned protocols. Multi-stage chromatographic techniques and orthogonal validation, including HPLC and mass spectrometry, ensure Glutathione Oxidized achieves consistent purity with minimal impurities or degradation products. This high level of purity guarantees reproducible experimental results across a wide range of laboratory applications.
Structural and Functional Integrity:
The disulfide-linked structure of Glutathione Oxidized is confirmed through MS and NMR analysis, ensuring proper molecular configuration for biochemical and mechanistic studies. Structural consistency is critical for accurately modeling redox reactions, thiol-disulfide exchange, and enzyme-substrate interactions in experimental systems.
Solubility and Handling Characteristics:
Lyophilized Glutathione Oxidized dissolves readily in research-grade water or mild buffer solutions, facilitating integration into analytical assays, enzymatic reactions, and multi-omic workflows. Handling guidelines recommend gentle mixing to preserve molecular integrity, and preparation should be conducted using sterile equipment to avoid contamination.
Storage and Stability:
The peptide remains stable under recommended storage conditions, retaining its chemical and structural integrity for long-term research use. Laboratories are advised to store aliquots in moisture-protected containers and minimize freeze–thaw cycles, which preserves peptide performance and reduces variability between experimental runs.
Packaging and Traceability:
Each vial of Glutathione Oxidized is individually labeled with batch number, COA reference, and storage information. Bulk orders can be customized with special labeling, aliquot sizes, or packaging tailored for institutional workflows. Complete documentation, including chromatograms, mass spectra, and QC notes, is provided with every shipment, supporting reproducibility and regulatory compliance.
Application Readiness:
The comprehensive specifications make High-purity freeze-dried peptide powder suitable for a variety of research applications, including oxidative stress modeling, enzymatic assays, redox potential evaluation, and multi-omic studies. The combination of high purity, validated solubility, and structural integrity ensures reliable experimental outcomes across diverse laboratory settings.

Mechanism of Action
Glutathione Oxidized functions as the disulfide form of glutathione (GSSG) and plays a central role in redox biology studies by serving as a substrate and modulator in enzymatic and chemical oxidation-reduction cycles. In controlled laboratory experiments, Glutathione Oxidized participates in reactions mediated by glutathione reductase, thiol-disulfide exchange processes, and redox-sensitive protein regulation. Its molecular structure, comprising two glutathione units linked by a disulfide bond, allows precise examination of oxidative conversion kinetics, intracellular redox balancing, and enzyme specificity under experimental conditions.
The peptide is commonly employed in assays assessing redox potential changes, reactive oxygen species scavenging, and modulation of antioxidant defense pathways. Researchers use High-purity freeze-dried peptide powder to probe the equilibrium between reduced (GSH) and oxidized glutathione in biochemical systems, mapping enzyme activity, cofactor interactions, and electron transfer reactions. Laboratory studies often monitor the generation or reduction of GSSG to understand glutathione-dependent regulatory mechanisms and the dynamics of oxidative stress signaling.
Furthermore, Glutathione Oxidized is used in combination with other redox-active compounds to investigate the specificity and kinetics of thiol-disulfide exchange reactions, protein S-glutathionylation, and peptide-mediated redox modulation. It provides a reproducible substrate for validating computational models of redox potential and for high-throughput screening assays in oxidative stress research. By controlling the oxidative environment with High-purity freeze-dried peptide powder, researchers can dissect mechanisms of cellular protection, enzyme function, and redox-mediated biochemical regulation without introducing human or animal use contexts.
Applications
Glutathione Oxidized (GSSG) is extensively used in laboratory research to study redox biology, oxidative stress regulation, and glutathione-dependent enzymatic processes. Its high purity and well-characterized structure make it a versatile reagent for biochemical assays, molecular studies, and multi-omic workflows. Researchers employ High-purity freeze-dried peptide powder to examine the balance between reduced (GSH) and oxidized glutathione, which is crucial for understanding cellular antioxidant defenses and thiol-disulfide homeostasis.
In biochemical research, Glutathione Oxidized is used to investigate enzyme kinetics, particularly for glutathione reductase and other oxidoreductases. It serves as a substrate to monitor redox cycling and enzymatic activity, enabling precise measurement of oxidative changes in controlled experimental conditions. Its stability and solubility support reproducible assays, including spectrophotometric, fluorometric, and chromatographic analyses, providing reliable data for laboratory investigations into redox-dependent mechanisms.
In molecular and cellular studies, High-purity freeze-dried peptide powder facilitates research into protein S-glutathionylation, redox-sensitive signaling pathways, and regulation of transcription factors influenced by oxidative stress. It is frequently applied in controlled in vitro systems, including cell-free models, cell extracts, and organotypic preparations, to simulate oxidative environments and study molecular interactions without human or animal administration. Researchers can use High-purity freeze-dried peptide powder to explore the effects of redox changes on metabolic pathways, protein folding, and stress-response signaling in laboratory settings.
Glutathione Oxidized is also employed in structural biology and computational modeling. Its predictable redox properties allow detailed analysis of disulfide bond formation, peptide-protein interactions, and thiol-disulfide dynamics. In silico simulations integrate Glutathione Oxidized to model oxidative conditions, predict enzyme-substrate behavior, and support the design of mechanistic experiments. These approaches enhance understanding of peptide-mediated regulation at molecular and systems levels.
In high-throughput and multi-omic research, High-purity freeze-dried peptide powder is compatible with transcriptomics, proteomics, metabolomics, and phosphoproteomics workflows. Researchers use it to map network-wide responses to oxidative changes, investigate antioxidant pathway regulation, and quantify biochemical signatures associated with peptide-mediated redox modulation. The peptide’s high purity and structural stability enable reliable integration into complex, sequential assays and cross-platform experimental designs.
Additionally, High-purity freeze-dried peptide powder is suitable for engineered laboratory systems, including microfluidic chips and organ-on-chip models. These platforms allow precise control of oxidative environments and dynamic redox gradients. High-purity freeze-dried peptide powder provides a stable, high-quality reagent for simulating physiological or stress conditions in vitro, supporting mechanistic studies of cellular signaling and peptide-mediated regulation.
In summary, the applications of Glutathione Oxidized span biochemical assays, molecular studies, multi-omic analyses, computational modeling, and advanced engineered systems. Its high purity, reproducibility, and compatibility with diverse laboratory models make it an indispensable reagent for investigating oxidative stress, redox homeostasis, and glutathione-dependent biological processes in research contexts.
Research Models
Glutathione Oxidized is compatible with diverse laboratory research models designed to examine redox processes, antioxidant defenses, and glutathione-dependent enzymatic activity.
Cell-Based Models: In vitro cell lines, including hepatocytes, neuronal cells, and engineered redox-sensitive reporter cells, allow controlled evaluation of oxidative stress responses and GSSG/GSH balance. Researchers can assess thiol-disulfide regulation, intracellular redox shifts, and downstream signaling pathways using high-precision fluorescence or spectrophotometric assays.
Biochemical Assays: Purified enzyme systems, reconstituted pathways, and chemical reaction models provide a defined environment for measuring glutathione reductase activity, disulfide exchange kinetics, and substrate specificity. High-purity freeze-dried peptide powder serves as a key reagent for quantitative analysis in these mechanistic setups.
High-purity freeze-dried peptide powder: In silico simulations of redox potential, peptide-mediated thiol-disulfide interactions, and pathway modeling rely on Glutathione Oxidized as a benchmark molecule. Molecular docking and dynamic simulations help predict enzymatic interactions and optimize experimental conditions.
Multi-Omic Platforms: Integration with transcriptomics, proteomics, and metabolomics allows researchers to correlate oxidative states with gene expression, protein modifications, and metabolic fluxes. High-purity freeze-dried peptide powder ensures reproducibility and accurate calibration across these complex datasets.
Engineered Systems: Microfluidic chips and organ-on-chip platforms simulate redox gradients and oxidative environments. Glutathione Oxidized provides controlled input for experiments evaluating stress responses, signaling networks, and peptide-mediated regulation without human or animal application.

Experimental Design Considerations
When designing laboratory experiments with Glutathione Oxidized, careful planning is essential to ensure reproducibility, data accuracy, and meaningful insights into redox biology. Researchers should consider peptide concentration, solvent compatibility, and buffer composition, as these factors influence solubility, stability, and reactivity in oxidative and enzymatic assays. Maintaining a controlled redox environment is critical, especially when studying thiol-disulfide exchange, glutathione-dependent enzymatic kinetics, or oxidative stress modulation.
Time-course studies with Glutathione Oxidized can reveal dynamic changes in redox potential, the conversion between reduced and oxidized glutathione, and the kinetics of enzymatic reactions such as glutathione reductase activity. Including appropriate controls—such as reactions without peptide, with reduced glutathione (GSH), or with known inhibitors—helps differentiate specific effects of the peptide from background oxidative fluctuations. Replicates are recommended to account for experimental variability and ensure statistical robustness.
In multi-step workflows or multi-omic studies, documentation of handling, preparation, and storage conditions is essential. Aliquoting Glutathione Oxidized into pre-measured volumes prevents repeated freeze–thaw cycles, which could compromise molecular integrity. When combining the peptide with other reagents, compatibility tests are advisable to avoid precipitation, degradation, or unintended redox reactions. Researchers may also consider temperature, pH, and ionic strength, as these parameters affect disulfide stability and enzymatic reaction rates.
Integration with computational modeling can guide experimental design by predicting redox potential changes, thiol-disulfide reaction kinetics, and enzyme-substrate interactions. Simulated data help optimize concentrations, incubation times, and assay conditions, minimizing trial-and-error and enhancing efficiency in laboratory studies. For high-throughput assays, automation protocols should incorporate peptide handling instructions to maintain reproducibility across multiple plates or experimental runs.
Quality control measures are critical. Using batch-verified High-purity freeze-dried peptide powder with accompanying COA and HPLC profiles ensures consistency across experiments. Researchers should periodically verify the peptide’s purity and structural integrity, especially in long-term studies. Detailed record-keeping of batch numbers, storage conditions, and experimental conditions supports reproducibility and facilitates troubleshooting if discrepancies arise.
Finally, experimental designs should anticipate downstream analyses, such as enzymatic activity measurement, redox potential quantification, or multi-omic integration. Planning sample collection, timing, and data recording ensures that the full spectrum of redox-related insights can be captured. Adherence to rigorous design principles allows Glutathione Oxidized to serve as a reliable tool in investigating oxidative stress, redox regulation, and glutathione-mediated pathways in research-only laboratory settings.
Laboratory Safety & Handling Guidelines
High-purity freeze-dried peptide powder should be handled in designated laboratory areas following institutional chemical safety protocols. Personnel must wear gloves, lab coats, and eye protection when handling the lyophilized powder. Work surfaces should be sanitized before and after experiments.
Store the peptide at −20 °C in moisture-protected containers. Avoid repeated freeze–thaw cycles. Prepare solutions using sterile techniques and high-purity buffers. Label all containers with concentration, batch number, and date. Follow institutional procedures for disposal of unused material, ensuring chemical waste regulations are observed. Maintain safety documentation, including COA and MSDS, for audit readiness and regulatory compliance.
Integration with Multi-Omic & Computational Studies
High-purity freeze-dried peptide powder plays a pivotal role in multi-omic and computational research approaches aimed at understanding cellular redox balance, oxidative stress, and glutathione-mediated biochemical pathways. Its high purity, stability, and well-characterized structure make it an ideal reagent for integrating transcriptomics, proteomics, metabolomics, and redox-sensitive assays in laboratory studies. Researchers can employ Glutathione Oxidized to correlate redox states with gene expression patterns, post-translational protein modifications, and metabolic fluxes, enabling a systems-level understanding of oxidative regulation.
In proteomic studies, Glutathione Oxidized is used to examine thiol-disulfide modifications, such as protein S-glutathionylation, which influence enzyme activity, signaling pathways, and stress response networks. By combining this peptide with high-resolution mass spectrometry, researchers can identify redox-sensitive protein targets and quantify oxidative modifications in experimental systems. Its consistent chemical quality ensures reproducibility across replicate analyses and multi-laboratory studies.
In metabolomics and redox metabolite profiling, Glutathione Oxidized serves as a reference standard for quantifying GSSG/GSH ratios, monitoring antioxidant capacity, and evaluating the effects of oxidative conditions on cellular metabolism. Coupled with LC-MS, NMR spectroscopy, or fluorometric detection, it provides precise calibration for experimental assays, enhancing accuracy in systems-level analysis of redox-dependent metabolites.
Transcriptomic integration involves assessing changes in gene expression driven by oxidative stress or altered redox potential. Using controlled experimental addition of Glutathione Oxidized, researchers can observe upregulation or suppression of antioxidant genes, redox-responsive transcription factors, and stress signaling pathways. This data supports computational modeling to predict network-level effects and dynamic responses in diverse cell types or biochemical systems.
Computational approaches, including molecular dynamics simulations, reaction kinetics modeling, and pathway network analysis, utilize Glutathione Oxidized as a reference compound to validate theoretical predictions and optimize experimental designs. In silico simulations help researchers anticipate thiol-disulfide exchange kinetics, enzyme-substrate interactions, and the impact of oxidative shifts on multi-step biochemical reactions. These models provide mechanistic insights, guide hypothesis-driven experiments, and reduce trial-and-error in laboratory workflows.
Additionally, High-purity freeze-dried peptide powder can be incorporated into integrative multi-omic studies, combining data across genomic, proteomic, metabolomic, and computational platforms. Its stability and reproducibility ensure that oxidative modulation experiments generate reliable cross-platform datasets. Researchers can link molecular-level observations to system-wide effects, elucidating redox regulation, metabolic adaptation, and peptide-mediated biochemical control with high confidence.
In summary, Glutathione Oxidized serves as a robust reagent for advanced laboratory research integrating multi-omic analyses and computational modeling. Its role in redox measurement, oxidative stress simulation, and enzyme-substrate characterization provides a comprehensive foundation for systems biology studies, network modeling, and mechanistic investigations of glutathione-dependent pathways in experimental research environments.

Keywords
GSSG, CAS 27025-41-8, redox peptide, oxidized glutathione, laboratory reagent, peptide research, high purity peptide, oxidative stress research, enzymatic assays, antioxidant pathway studies
Shipping Guarantee
All shipments of High-purity freeze-dried peptide powder are transported in validated temperature-controlled packaging (2–8 °C) with moisture and tamper protection. Each order includes COA, MSDS, batch documentation, and real-time tracking. The packaging is tested for long-distance global transport to maintain peptide integrity.
Trade Assurance
Factory-direct, GMP-aligned production ensures consistent purity and reproducible batch quality. OEM customization, bulk supply, and institutional packaging options are available. Documentation accompanies every order to support laboratory compliance and traceability.
Payment Support
Accepted methods include bank transfer, corporate accounts, major credit cards, PayPal, and cryptocurrency for approved partners. Bulk or wholesale orders may access flexible terms. All transactions follow secure, auditable international procurement standards.
Disclaimer
is for laboratory research use only.
Not for human or veterinary use.
All handling must follow institutional biosafety protocols, chemical hygiene plans, and regulatory standards.
References
Jones, D.P., & Go, Y.M. (2010). Redox compartmentalization and cellular signaling. Journal of Biological Chemistry. https://pubmed.ncbi.nlm.nih.gov/20086287
Townsend, D.M., Tew, K.D., & Tapiero, H. (2003). The importance of glutathione in human disease. Biomedicine & Pharmacotherapy. https://pubmed.ncbi.nlm.nih.gov/14629973
Meister, A., & Anderson, M.E. (1983). Glutathione. Annual Review of Biochemistry. https://pubmed.ncbi.nlm.nih.gov/6136434
Lu, S.C. (2013). Glutathione synthesis. Biochimica et Biophysica Acta (BBA) – General Subjects. https://pubmed.ncbi.nlm.nih.gov/23098688
Wu, G., Fang, Y.Z., et al. (2004). Glutathione metabolism and functions. Journal of Nutrition. https://pubmed.ncbi.nlm.nih.gov/14988470
Additional information
| Weight | 0.6 kg |
|---|---|
| Dimensions | 23 × 26 × 23 cm |
1. What is Glutathione Oxidized used for in research?
Glutathione Oxidized is primarily used to study oxidative stress, redox biology, and glutathione-dependent enzymatic reactions. It provides a stable and high-purity reagent to evaluate redox potential, enzyme kinetics, and thiol-disulfide homeostasis in laboratory experiments.
2. How should Glutathione Oxidized be stored?
Store at −20 °C in tightly sealed, light-protected containers to maintain stability. Avoid repeated freeze–thaw cycles and protect from moisture to ensure consistent experimental results.
3. Is Glutathione Oxidized compatible with multi-omic studies?
Yes, it integrates seamlessly into transcriptomics, proteomics, metabolomics, and redox-sensitive assays. Researchers use it to correlate oxidative states with gene expression, protein modifications, and metabolic profiles.
4. What purity does Glutathione Oxidized have?
Each batch is verified to be ≥98% purity by HPLC. This high purity ensures reproducible results in biochemical, enzymatic, and analytical research workflows.
5. Can Glutathione Oxidized be purchased in bulk?
Yes, bulk and OEM packaging options are available for research institutions and laboratories. Custom labeling and vial sizes can be provided upon request.
6. Does the product come with quality documentation?
Yes, each shipment includes a Certificate of Analysis (COA), MSDS, batch QC report, and chromatograms to support traceability and regulatory compliance.
7. Can Glutathione Oxidized be used in enzymatic assays?
Absolutely. It is commonly employed in assays to evaluate glutathione reductase activity, thiol-disulfide exchange reactions, and other redox-dependent enzymatic pathways.
8. Is technical support available for handling and experimental use?
Yes, technical assistance is provided for solubility, preparation, and integration into laboratory protocols to ensure optimal results.
9. Can Glutathione Oxidized be integrated into computational modeling?
Yes, it serves as a benchmark for molecular dynamics simulations, redox potential modeling, and enzyme-substrate interaction studies.
10. Is Glutathione Oxidized suitable for cell-based assays?
Yes, it can be used in cell-free systems or cell extracts to study oxidative responses, but it is strictly for research use and not for human or veterinary applications.
11. How should I prepare Glutathione Oxidized solutions?
Dissolve gently in sterile water or suitable buffers under mild conditions to maintain molecular integrity. Avoid harsh conditions that could disrupt the disulfide structure.
12. What precautions should be taken during handling?
Use appropriate PPE, including gloves and eye protection. Work in designated laboratory areas and follow chemical hygiene protocols to prevent contamination.

















Reviews
There are no reviews yet.