No products in the cart.
Lamivudine and Tenofovir Tablets (Taidou) – AR Grade Factory low price wholesale
$1.00
Combination antiviral tablets containing lamivudine and tenofovir—Lam-Ten formula—for HIV research and antiviral pharmacology studies. Pack of 30 tablets. Supports wholesale and retail.?For wholesale prices, other specifications and uses, please consult our staff?
Description
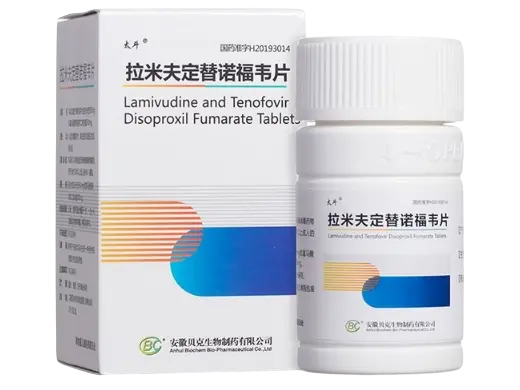
Lamivudine and Tenofovir Tablets (Taidou) provide a fixed-dose combination of two antiviral agents commonly used in HIV research. Manufactured by Anhui Becker Biopharmaceutical Co., Ltd., these tablets hold NMPA approval (H20193014) and are intended solely for laboratory and nonclinical research purposes.
Formulated for consistency and precision, these tablets are ideal for use in in vitro studies, pharmacokinetic modeling, and antiviral mechanism research.
Lamivudine and Tenofovir Tablets Product Parameters
| Parameter | Details |
|---|---|
| Product Name | Lamivudine & Tenofovir Tablets (Taidou) |
| Active Ingredients | Lamivudine + Tenofovir Disoproxil Fumarate |
| Strength | Combination dose per tablet (manufacturer spec) |
| Quantity | 30 tablets per bottle |
| Dosage Form | Oral tablets |
| Approval Number | H20193014 (China NMPA) |
| Product Code | 86979267000118 |
| Manufacturer | Anhui Becker Biopharmaceutical Co., Ltd. |
| Barcode | 6948163534243 |
| Storage Conditions | Store at 20–25?°C, protected from moisture |
| Intended Use | Laboratory research only |
Lamivudine and Tenofovir Tablets Mechanism of Action
Lamivudine and Tenofovir inhibit the HIV reverse transcriptase enzyme, blocking viral replication. This combination is widely studied in laboratory antiviral research.
Lamivudine and Tenofovir Tablets Potential Side Effects (Research Context)
Mild gastrointestinal effects in vivo models
Possible renal function changes under extended dosing
Headache or dizziness in animal studies
Disclaimer
This product is for laboratory research use only. It is not intended for human or veterinary therapeutic use. Buyers must ensure compliance with all applicable laws and regulations.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 23 × 36 × 23 cm |
Q1: What is the intended use of Lamivudine and Tenofovir Tablets (Taidou)?
These tablets are intended solely for laboratory research and nonclinical antiviral studies.
Q2: Can they be used in clinical treatment?
No. This product is not approved for therapeutic or diagnostic use.
Q3: How should they be handled in research?
Use under controlled lab conditions with appropriate dosing based on experimental design.
Q4: Are there any known risks in lab settings?
Follow standard safety protocols; renal and mitochondrial effects are reported in vivo with extended dosing.
Q5: Is bulk purchasing available?
Yes, wholesale orders are supported for laboratory supply.








Reviews
There are no reviews yet.