No products in the cart.
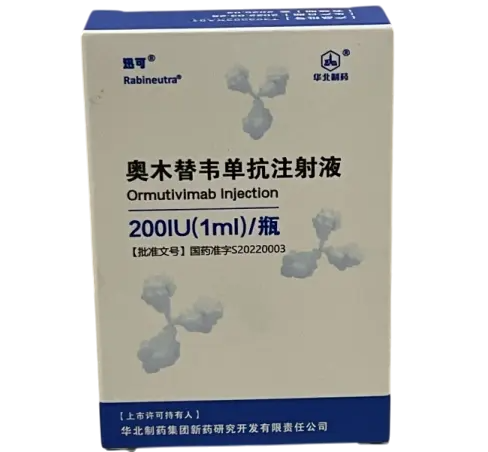
Ormutivimab Injection 200?IU (1?ml) Vial – Wholesale & Retail (For Research Use Only)
$1.00
Ormutivimab Injection is a recombinant human anti?rabies monoclonal antibody (rhRIG), 200?IU in 1?ml vial. Made by North China Pharmaceutical / Huabei Co., approved under licence S20220003. Used in vaccine and rabies PEP research, neutralizing studies and pharmacodynamics. Wholesale & retail supported. For laboratory research use only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
Ormutivimab (also known as NM57) is a fully human IgG1 monoclonal antibody produced in CHO cells, specifically neutralizing rabies virus. It is the first approved recombinant human rabies immunoglobulin (rhRIG) in China, offering consistent potency and safety compared to HRIG in clinical trials. In research contexts, Ormutivimab is used to model post?exposure prophylaxis, rabies virus neutralization, and pharmacokinetics of antibody interventions. For laboratory research use only.
Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Ormutivimab Injection |
| Synonyms | NM57; rhRIG; recombinant human rabies immunoglobulin |
| Strength | 200?IU per vial (1?ml) |
| Dosage Form | Injectable vial |
| Packaging | 1 vial per box |
| Manufacturer | North China Pharmaceutical / Huabei Biotech Co., Ltd. |
| Approval Number | S20220003 |
| Drug Standard Code | 86902700000215 |
| Barcode | (not yet assigned) |
| CAS Number | N/A (monoclonal antibody complex) |
| Molecular Type | Fully human IgG1 (~150?kDa) |
Mechanism of Action & Research Applications
Ormutivimab binds the rabies virus glycoprotein, neutralizing diverse rabies strains with high potency (198–1487 IU/mL). It prevents virus entry and enhances passive immunity rapidly post-exposure. It is ideal for research involving rabies PEP modeling, comparative studies with HRIG, vaccine-antibody interaction, and early immune response kinetics.
Side Effects (For Reference Only in Research Models)
Clinical trials reported generally mild to moderate injection site and systemic reactions, similar rates to HRIG, with favorable safety and neutralizing profiles at doses 20?IU/kg and 40?IU/kg combined with vaccine administration.
Disclaimer
Ormutivimab Injection is strictly intended for laboratory research use only. Not for human or veterinary therapeutic or diagnostic use.
Additional information
| Weight | 0.5 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |










Reviews
There are no reviews yet.