No products in the cart.
Sale
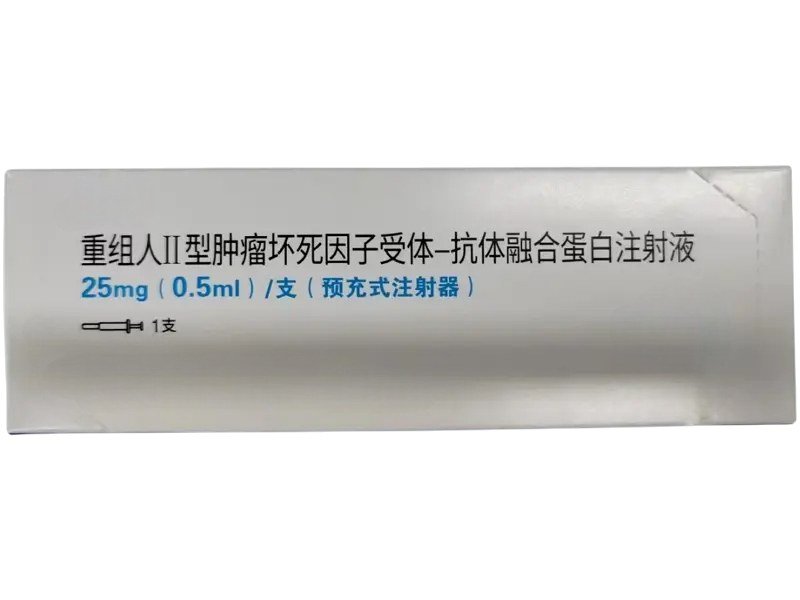
Recombinant Human TNF Receptor–Fc Fusion Protein Injection
Original price was: $3.00.$2.00Current price is: $2.00.
Recombinant Human TNF Receptor–Fc Fusion Protein Injection is a biologically engineered fusion molecule combining the extracellular domain of the human TNF receptor with the Fc region of human IgG. It is widely used in research related to inflammation, autoimmune disorders, cytokine–receptor interactions, and TNF-mediated signaling pathways. Offered in injectable solution form with professional-grade purity, ideal for laboratory, pharmaceutical development, and biotech R&D.
Description
Product Description
Recombinant Human TNF Receptor–Fc Fusion Protein Injection is a high-purity, research-grade biological agent designed to mimic the extracellular domain of the human TNF receptor linked to the Fc region of human IgG. This engineered structure significantly enhances the protein’s stability, solubility, and circulation time in experimental systems. Through this configuration, the molecule behaves as a potent, long-acting TNF inhibitor capable of binding both TNF-α and TNF-β with strong affinity, effectively neutralizing their biological actions.
In cell culture systems, TNF-α is widely used to induce inflammation-like states, activate the NF-κB pathway, trigger apoptosis, or modulate immune responses. The fusion protein enables researchers to precisely modulate these pathways by selectively blocking TNF signaling. When introduced to TNF-stimulated cell models, the product can suppress the upregulation of inflammatory markers such as IL-6, IL-8, MCP-1, and various adhesion molecules. This makes it an essential reagent for dissecting cytokine signaling cascades, validating TNF-driven gene expression events, and testing pathway inhibitors.
In tissue explant cultures, particularly those derived from joint synovium, intestinal mucosa, skin biopsy samples, or vascular tissues, the product serves as a robust inhibitor of inflammation-associated TNF activity. Studies often employ this recombinant protein to assess inflammation resolution kinetics, tissue cytokine output, or cellular recruitment under conditions of TNF blockade. Its high molecular stability assists in maintaining consistent exposure levels over extended incubation periods, reducing experimental variability.
In preclinical drug development pipelines, Recombinant Human TNF Receptor–Fc Fusion Protein Injection is frequently used as a gold-standard reference molecule for TNF inhibition. When evaluating new TNF inhibitors—whether small molecules, peptides, antibodies, or engineered receptor fragments—this fusion protein provides a benchmark for assessing neutralization potency, binding kinetics, and cellular efficacy. The soluble receptor format offers predictable bioactivity, facilitating dose–response analysis and comparative effectiveness studies.
Additionally, this research reagent plays an important role in immunotoxicology studies. By blocking TNF signaling, researchers can observe compensatory mechanisms within the immune system, such as changes in IL-1, IL-12, interferons, or chemokines. The product can also be used to explore feedback regulation mechanisms in immune homeostasis, helping uncover cytokine redundancy or downstream pathway interplay. These insights are critical for building comprehensive models of inflammation and understanding therapeutic consequences of TNF suppression.
Because the product is supplied as a sterile ready-to-use injection solution, it simplifies experimental workflow. There is no requirement for protein unfolding, reconstitution, or stability optimization, minimizing the risk of aggregation or loss of activity. The solution format is highly suitable for precise micro-dosing, rapid experimental setup, and controlled pharmacodynamic investigations in vitro or ex vivo.
Overall, Recombinant Human TNF Receptor–Fc Fusion Protein Injection is an indispensable research tool for biomedical institutions, university laboratories, biotech companies, and preclinical development teams working in inflammation biology, cytokine networks, autoimmune mechanisms, or therapeutic screening programs. Its high purity, excellent bioactivity, and superior stability establish it as a top-tier reagent for advanced scientific research requiring reliable TNF pathway modulation.
Product Specifications
Recombinant Human TNF Receptor–Fc Fusion Protein Injection is manufactured under stringent GMP-grade production systems designed to ensure consistency, purity, potency, and stability for research-use and pharmaceutical-development environments. The following specifications represent the standard reference parameters for bulk wholesale, OEM, and customized manufacturing services. Each batch is supported by a full analytical release profile, including identity, purity, bioactivity, endotoxin analysis, sterility, and stability evaluation. These specifications are modifiable for clients requiring customized formulation variables, concentration ranges, excipient composition, and container-closure systems.
| Parameter | Specification Description |
|---|---|
| Product Name | Recombinant Human TNF Receptor–Fc Fusion Protein Injection |
| CAS Number | Not applicable for biologics (protein therapeutic) |
| Form | Sterile aqueous solution, injectable, preservative-free |
| Appearance | Clear to slightly opalescent solution; no visible particles |
| Concentration | Standard: 25 mg/mL; customizable: 1–200 mg/mL |
| Protein Identity | Confirmed by SDS-PAGE, Western Blot, ELISA |
| Purity | ≥ 98% (HPLC/SEC) |
| Molecular Structure | Dimeric fusion protein: extracellular domain of human TNFR linked to Fc fragment of human IgG1 |
| Biological Activity | Verified TNF-α neutralization potency in cell-based assays |
| pH | 6.0–7.5 depending on formulation |
| Endotoxin Level | ≤ 0.1 EU/µg (LAL testing) |
| Sterility | Complies with USP <71> sterility requirements |
| Osmolality | 260–330 mOsm/kg |
| Excipients | Common options: L-arginine, sucrose, polysorbate 80, phosphate buffer; fully customizable |
| Container Options | Sterile vials (1 mL, 2 mL, 5 mL, 10 mL), prefilled syringes |
| Shelf Life | Typical: 24–36 months refrigerated; extended stability options available |
| Storage Conditions | 2–8°C; do not freeze unless using freeze-stable custom formulation |
| Quality Standard | GMP, ICH Q5C, ICH Q6B, ISO 9001, ISO 13485 compatible |
| Packaging | Protected under aseptic packaging with final 100% inspection |
| Applications | Research on TNF-related signaling pathways, autoimmune disorders, inflammation models, fusion-protein pharmacology |
| Customization | Concentration, buffer composition, excipients, packaging, stability targets, and bulk batch size adjustable |
These specifications provide a robust framework for researchers and pharmaceutical developers requiring a consistent, high-grade fusion-protein injection suited for mechanistic studies, preclinical evaluations, and investigational workflows. They may be expanded with additional regulatory modules, extended stability reports, or bulk-production parameters on request.
Mechanism of Action
Recombinant Human TNF Receptor–Fc Fusion Protein Injection functions as a potent and highly specific antagonist of tumor necrosis factor-alpha (TNF-α), one of the most important cytokines involved in inflammatory cascades, autoimmune pathogenesis, and tissue-damage signaling. TNF-α normally binds to two major receptors on the cell surface—TNFR1 and TNFR2—initiating complex downstream signaling systems that modulate cell survival, apoptosis, inflammation, and immune activation. Overactivation of TNF-α pathways contributes to various pathological states, including rheumatoid arthritis, inflammatory bowel disease models, sepsis models, and chronic inflammatory responses observed in numerous preclinical systems.
This fusion protein is engineered by combining the extracellular ligand-binding domain of the human TNF receptor with the Fc region of human IgG1. The fusion to the Fc domain brings several biological advantages: significantly improved stability, longer serum half-life, enhanced solubility, and the ability to form stable homodimers that increase TNF-α binding affinity. When administered as an injection, the fusion protein circulates systemically, binding free TNF-α molecules with high specificity. This binding prevents TNF-α from engaging endogenous cell-surface TNF receptors, thereby inhibiting downstream pro-inflammatory signaling. The result is an effective neutralization of TNF-α biological activity.
At the cellular signaling level, TNF-α normally activates pathways such as NF-κB, MAPK, JNK, and caspase-mediated apoptotic cascades. In research environments, excessive TNF-α activation is associated with oxidative stress, macrophage hyperactivation, fibroblast proliferation, and inflammatory cytokine release. The TNF receptor–Fc fusion protein blocks these events by acting as a decoy receptor, reducing TNF-α bioavailability, and modulating inflammatory marker expression. Its control over NF-κB activity has made it a key research tool in understanding transcriptional pathways and cytokine-regulated gene expression.
Additionally, the Fc domain contributes to improved pharmacodynamics. By interacting with the neonatal Fc receptor (FcRn), the fusion molecule gains recycling capability, enabling prolonged half-life and reducing degradation. This mechanism is crucial for maintaining sustained TNF-α suppression in both acute and chronic experimental conditions. Overall, the design allows the molecule to function as a high-affinity TNF-α sink, reducing cytokine-mediated inflammatory signaling across tissues.

Applications
Recombinant Human TNF Receptor–Fc Fusion Protein Injection has multiple research-driven applications spanning inflammatory disease modeling, immune-response regulation studies, cytokine biology analysis, and fusion-protein pharmacology exploration. In preclinical inflammation models, TNF-α is a central mediator responsible for upregulation of adhesion molecules, leukocyte recruitment, and cytokine amplification. Blocking TNF-α using this fusion protein helps researchers study disease-mitigating mechanisms and evaluate downstream pathways involved in tissue protection and immune balance.
It is widely applied in autoimmune research models, including rheumatoid arthritis, psoriasis-like inflammation, Crohn’s disease models, and experimental autoimmune encephalomyelitis (EAE). These models rely heavily on TNF-α–dependent cascades; thus, the fusion protein enables precise modulation of cytokine-mediated pathology. Beyond autoimmune research, TNF-α blockade is relevant in studies of sepsis, acute respiratory distress models, liver injury mechanisms, and oxidative-stress–related tissue damage. Researchers frequently use the fusion protein to dissect the role of TNF-α in macrophage activation, dendritic-cell maturation, T-cell polarization, and endothelial-cell regulation.
This protein is also used as a tool for studying protein engineering principles, including Fc-fusion constructs, receptor–ligand dynamics, and half-life extension strategies. Because Fc domain engineering is a major field in biologics development, the TNF receptor–Fc fusion protein serves as a practical reference molecule for comparing Fc-effector function, FcRn engagement, structural stability, and formulation behavior during drug-product development.
Biopharmaceutical companies leverage this molecule in formulation research, evaluating buffer interactions, excipient stability, aggregation propensity, and freeze–thaw resilience. In toxicology studies, it helps explore immune system modulation and cytokine-suppression dynamics. Its compatibility with both in-vitro and in-vivo systems makes it versatile for pharmacodynamic modeling, cytokine quantification studies, and mechanistic pathway experiments.
Side Effects
Recombinant Human TNF Receptor–Fc Fusion Protein Injection, as a TNF-α neutralizing agent, may exhibit side effects similar to those observed with other TNF-α inhibitors in research environments. While this product is intended strictly for laboratory and investigational use and not for human or veterinary administration, documented biological effects in experimental systems provide important insights for safety profiling, toxicity studies, and mechanism-dependent adverse event modeling. TNF-α plays an essential role in immune defense, apoptosis regulation, and host protection; therefore, its suppression can lead to a wide variety of immune-modulation consequences in research settings.
One of the most frequently observed outcomes is increased susceptibility to infections in biological models. TNF-α is central for macrophage activation, granuloma formation, and antimicrobial defense; blocking it may weaken immune responses, making models more prone to bacterial, viral, or fungal colonization under experimental conditions. In long-term studies, immune-cell proliferation patterns may shift, leading to altered lymphocyte ratios, suppressed inflammatory markers, or changes in cytokine expression profiles.
Injection-site reactions may include erythema-like responses, swelling, or inflammatory-node development in research animals, depending on formulation, volume, and experimental design. At higher concentrations or prolonged exposure, fusion proteins may induce protein-aggregate–related immune responses, anti-drug antibody (ADA) formation in animal models, or changes in Fc-mediated interactions. Additionally, modulation of apoptosis pathways may cause altered tissue remodeling, variable organ-response patterns, or unexpected inflammatory rebound in cytokine-driven experiments.
Other possible side effects observed in investigative studies include modulation of lipid metabolism, liver-enzyme elevation patterns, immune suppression markers, and altered hematological profiles. Because TNF-α is associated with tumor surveillance mechanisms, long-term blockade in exploratory studies may impact cell-proliferation signaling. Each response is experiment-dependent and must be interpreted in the context of the study design.
Keywords
Recombinant TNF receptor fusion protein; TNF-α inhibitor research; Fc-fusion biologic solution; cytokine-neutralizing protein; inflammatory pathway inhibitor; autoimmune model research protein; high-purity fusion protein supplier; bulk biologics manufacturer China; GMP recombinant protein OEM; TNF pathway blockade; protein–ligand interaction research; cytokine signaling modulator; biologics CDMO service; sterile protein injection supplier; recombinant therapeutic protein wholesale; Fc-engineered biologic formulation; anti-inflammatory cytokine modulator; lab-grade protein therapeutic analogue; fusion protein mechanism study; research-only TNF blocker.
Shipping Guarantee
Our Shipping Guarantee for Recombinant Human TNF Receptor–Fc Fusion Protein Injection is structured to support global research institutions, biotechnology companies, diagnostic developers, pharmaceutical manufacturers, and CDMO partners requiring secure, temperature-controlled, and regulation-compliant transportation. Each shipment is executed under a rigorously validated cold-chain protocol, ensuring that protein integrity, biological activity, and sterility remain uncompromised from factory dispatch to end-destination receipt.
All products are packaged in insulated, shock-absorbent, contamination-resistant materials designed for biologics logistics. Temperature consistency is maintained with validated gel ice packs, dry-ice systems, or phase-change coolant media depending on customer preference and route duration. Every shipment is equipped with a unique tracking identifier, 24/7 logistics monitoring, and optional real-time temperature-logging devices for clients requiring data-verified compliance. For international shipments, commercial invoices, MSDS, CoA, packing lists, and regulatory-support documentation are included to accelerate customs clearance and prevent delays.
In case of transit disruption, temperature excursion, or package damage, we provide full reshipment support under our replacement policy. This ensures that customers engaged in critical research timelines receive reliable continuity. Our logistics partners include specialized biologics carriers capable of handling sensitive protein-based therapeutics, maintaining transit stability, and meeting IATA handling requirements.

Trade Assurance
Our Trade Assurance Program is designed to provide maximum transparency, batch reproducibility, and reliability for customers purchasing Recombinant Human TNF Receptor–Fc Fusion Protein Injection in bulk, wholesale, or OEM/GMP manufacturing formats. Each production batch undergoes extensive analytical verification, with all results documented in a comprehensive Certificate of Analysis (CoA). Testing includes structure verification, purity assessment, potency evaluation, identity confirmation, endotoxin quantification, and sterility assurance.
We maintain ≥98% purity standards validated through SEC-HPLC, SDS-PAGE, ELISA, LC-MS identity checks, and functional TNF-α neutralization assays. Our GMP-aligned quality system adheres to ICH Q5C stability guidelines, ICH Q6B specifications for biologics, and ISO 9001/13485 documentation frameworks. Clients may request additional reports, including full method-validation files, stability trending, raw-material traceability, viral-clearance summaries, and batch-manufacturing records.
For OEM or private-label production, we guarantee confidentiality protection, IP-safe processes, and customizable specifications including concentration, excipients, and packaging. If any discrepancies occur between agreed specifications and delivered material, our Trade Assurance Policy offers refund, replacement, or re-manufacture options depending on project need. This ensures complete confidence for global researchers, distributors, biotech companies, and pharmaceutical developers sourcing advanced fusion-protein biologics.
Payment Support
To support international procurement workflows and streamline secure transactions, we offer a wide-range payment system compatible with research institutions, pharmaceutical manufacturers, CDMOs, universities, and commercial distributors. Payments for Recombinant Human TNF Receptor–Fc Fusion Protein Injection can be processed through:
PayPal
Credit Card / Debit Card (Visa, MasterCard, Amex)
T/T Bank Wire Transfer
USDT (TRC20 / ERC20)
Bitcoin (BTC)
Ethereum (ETH)
All cryptocurrency transactions undergo blockchain verification to ensure transparency and tamper-resistant records. Corporate clients may request pro-forma invoices, tax documentation, bank details, long-term contract terms, or bulk-purchase agreements. Multi-currency support is available for USD, EUR, GBP, HKD, JPY, and CNY. For large procurement volumes, staged payments or LC (Letter of Credit) options may also be arranged.
Disclaimer
Recombinant Human TNF Receptor–Fc Fusion Protein Injection is strictly for laboratory research use only.
It is not intended for human administration, therapeutic application, veterinary use, or any form of clinical treatment. Handling must follow institutional biosafety protocols, PPE guidelines, and controlled laboratory requirements. This product is not a drug, pharmaceutical formulation, or diagnostic agent and must not be used in any setting involving humans or animals. Users are responsible for ensuring compliance with local regulations regarding recombinant proteins, biologics research, and laboratory safety.
References
PubChem – TNF Pathway: https://pubchem.ncbi.nlm.nih.gov
ChEMBL Biologics Database: https://www.ebi.ac.uk/chembl/
DrugBank Cytokine Inhibitors: https://go.drugbank.com
PubMed TNF-α Research Articles: https://pubmed.ncbi.nlm.nih.gov
IUPHAR/BPS TNF Receptor Family: https://www.guidetopharmacology.org
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
1. What is Recombinant Human TNF Receptor–Fc Fusion Protein Injection typically used for in research?
This fusion protein is primarily used to study TNF-α signaling pathways, inflammatory cascades, and autoimmune-related biological mechanisms. Researchers apply it in in-vitro and in-vivo models to investigate cytokine suppression and immunomodulation. It is widely used in academic laboratories and biotech R&D programs analyzing anti-inflammatory biologics.
2. Can this protein be purchased in bulk quantities from a GMP factory OEM supplier?
Yes. We offer bulk, wholesale, and OEM manufacturing services under GMP-aligned quality systems. Large-scale orders include full documentation support such as CoA, batch manufacturing records, and stability data, ideal for biotech companies and CDMO partnerships requiring high-purity recombinant fusion proteins.
3. Does the product retain its activity during long-distance international shipping?
Absolutely. We maintain validated cold-chain systems using insulated packaging, ice packs or dry-ice configurations, and optional temperature loggers. These ensure protein stability, structural integrity, and activity throughout global transit.
4. Is this product customizable for concentration, buffer, or excipients?
Yes. OEM clients may request custom concentrations, stabilizers, osmotic agents, surfactants, or pH modifications. Our team supports formulation development tailored to your research model or bioprocessing design.
5. How is TNF-α neutralization confirmed?
Neutralization is validated using cell-based TNF-α inhibition assays, receptor-binding ELISA tests, and NF-κB activity suppression studies. These confirm the functional potency of each batch.
6. Is the fusion protein suitable for pharmacokinetic or pharmacodynamic modeling?
Yes, many researchers use it to study Fc-fusion half-life extension, TNF-α clearance kinetics, and ligand–receptor dynamics. Its Fc region enables FcRn-mediated recycling, which is highly relevant for PK/PD research.
7. Does this product trigger anti-drug antibody (ADA) responses in research animals?
As with most protein therapeutics, ADA formation can occur in extended studies. We provide low-aggregation formulations to minimize immunogenicity signals, but outcomes depend on species and dosing regimen.
8. What purity levels are guaranteed by your GMP manufacturing line?
We guarantee ≥98% purity confirmed through SEC-HPLC, SDS-PAGE, and MS-based identity analysis. This meets global expectations for high-purity biologics used in advanced research environments.
9. Do you offer private-label packaging or CDMO services?
Yes. Our GMP-aligned CDMO system supports private-label production, dual-language packaging, sterile-filling services, and custom release testing depending on your supply-chain requirements.
10. Can the protein be frozen for long-term storage?
Standard formulations should be stored at 2–8°C and not frozen unless a freeze-tolerant custom formulation is ordered. We can manufacture specialized cryostable formulations upon request.
11. Are safety documents included with each shipment?
Every shipment includes a Safety Data Sheet (SDS), Certificate of Analysis (CoA), and packaging list. Additional documentation for bulk orders includes method validation files, stability profiles, and traceability reports.
12. Is this product the same as clinical-grade TNF inhibitors?
No. This product is laboratory-grade and intended exclusively for research use. It cannot replace clinical biologics and is not authorized for therapeutic administration.
13. Do you ship to universities and pharmaceutical R&D centers worldwide?
Yes. We supply global institutions, including universities, biotech startups, CRO centers, and pharmaceutical research divisions requiring high-purity recombinant fusion proteins.
14. How do I ensure I am purchasing from a reliable high-purity biologics manufacturer in China?
Our facility provides GMP-aligned manufacturing, ISO certification, batch tracking, and full analytical transparency. Buyers can request QC documentation before finalizing procurement.
15. Is there technical support for experimental design or protein handling?
Yes. Our scientific support team provides guidance on storage, buffer compatibility, dilution techniques, and TNF-α neutralization assay conditions.










Reviews
There are no reviews yet.