No products in the cart.
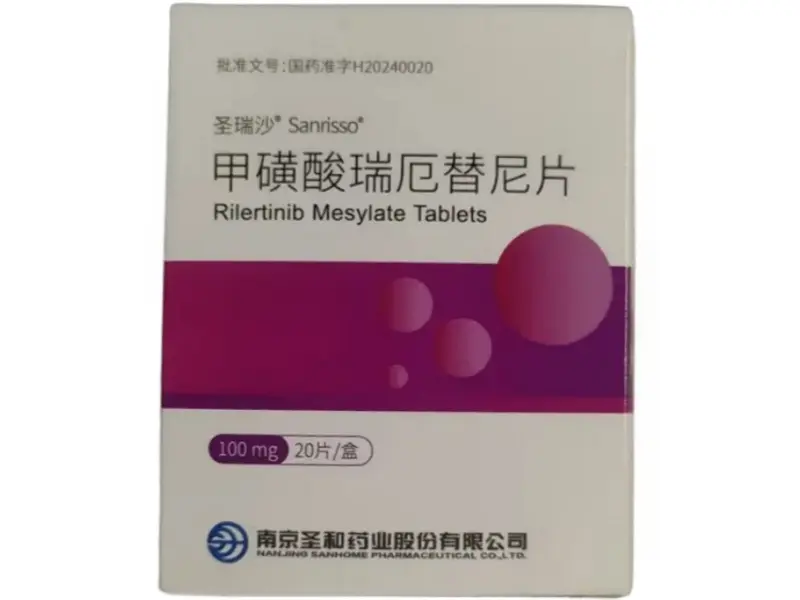
Rilertinib Mesylate Tablets (Sanrisso) – 100mg
$1.00
Rilertinib mesylate (Sanrisso), developed by Nanjing Sanhome Pharmaceutical Co., Ltd., is a third-generation EGFR-TKI approved by China’s NMPA (Approval No. H20240020; Drug Code: 86901583000930). Each bottle contains 20 tablets of 100mg each. Strictly for laboratory research use only—not for clinical or human use.
Description
Rilertinib Mesylate Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Rilertinib Mesylate Tablets (Sanrisso) |
| Dosage | 100mg per tablet × 20 tablets per bottle |
| Formulation | Oral film-coated tablet |
| Approval No. | China NMPA H20240020 |
| Drug Code | 86901583000930 |
| Manufacturer | Nanjing Sanhome Pharmaceutical Co., Ltd. |
| Intended Use | Laboratory research only—Not for human or veterinary use |
Overview & Mechanism
Rilertinib mesylate (Sanrisso®) is a third-generation, irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) that targets activating mutations and the T790M resistance mutation in non-small cell lung cancer (NSCLC). It exhibits potent anti-tumor activity against EGFR-mutant NSCLC cell lines and tumor models.
Research & Clinical Context
China NMPA Approval (Feb 2025): Approved for patients with EGFR T790M mutation-positive advanced or metastatic NSCLC after progression on prior EGFR-TKI therapy.
Phase III Trials: Demonstrated superior progression-free survival (PFS) compared to gefitinib in EGFR-mutant NSCLC patients.
Research Applications
Ideal for:
EGFR pathway and T790M-resistance mechanisms
In vitro and in vivo NSCLC models
Drug resistance and combination therapy studies
Pharmacokinetic and pharmacodynamic profiling
Handling & Storage
Storage: Store at 20–25?°C, sealed and protected from light and moisture.
Handling: Use appropriate laboratory personal protective equipment (PPE); avoid ingestion or inhalation.
References
NMPA approval announcement (Feb 19, 2025)
Phase III trial results comparing rilertinib vs gefitinib
Drug mechanism and development synopsis
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 26 × 25 × 26 cm |










tinknocke –
The logistics was very fast, I received it in 8 days