Teriparatide Injection (Zhengu) 20??g – Low price wholesale comparison
$2.00
Teriparatide Injection (Zhengu) contains recombinant human PTH (1-34) at 20??g/dose in 80??L, provided in a 2.4?mL vial (5 vials per box). Manufactured by Shanghai United Cell Biotechnology Co., Ltd. Approved under NMPA S20240005. Strictly for laboratory research use only.?Please consult staff for other specifications and uses?
描述
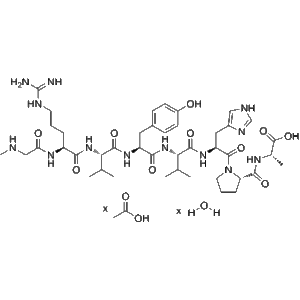
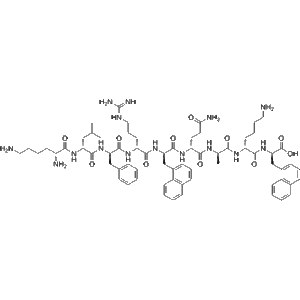
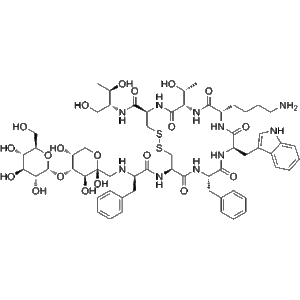
Teriparatide is a recombinant peptide, representing the first 34 amino acids of human parathyroid hormone. It has a robust anabolic effect on bone through intermittent PTH receptor activation. Zhengu provides 20??g doses in pre-filled vials, each containing 2.4?mL for precise subcutaneous or intravenous delivery—suitable for investigative studies in bone metabolism, osteogenesis, and biomarker analysis.
Developed by Shanghai United Cell Biotechnology Co., Ltd., recognized for biosimilar PTH products . NMPA approval number S20240005, drug code 86900719000097.
?? For laboratory research use only. Not for clinical, therapeutic, or veterinary application.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Teriparatide Injection (Zhengu) |
| Generic Name | Teriparatide (rhPTH 1?34) |
| CAS Number | 52232?67?4 |
| Molecular Formula | C???H???N??O??S? |
| Molecular Weight | ~4,117.8?Da |
| Dosage Form | Recombinant peptide injection |
| Strength | 20??g per 80??L dose in 2.4?mL vial |
| Packaging | 5 vials per box |
| Approval Number | S20240005 (China NMPA) |
| Product Code | 86900719000097 |
| Manufacturer | Shanghai United Cell Biotechnology Co., Ltd. |
| Storage Conditions | Store at 2–8?°C; protect from light and avoid freezing |
| Intended Use | Laboratory research only |
Mechanism & Research Applications
Teriparatide mimics intermittent PTH exposure, stimulating osteoblast differentiation and increasing bone formation markers while promoting bone remodeling. It’s utilized in studies such as:
-
In vitro osteoblast and osteocyte assays
-
Bone mineral density and fracture healing models
-
Biomarker quantification (e.g., P1NP, BSAP)
-
Preclinical osteoporosis and skeletal regeneration studies
Teriparatide Side Effects (Research Context)
Preclinical data and clinical analogues indicate potential effects:
-
Transient hypercalcemia and phosphaturia
-
Injection-site discomfort and mild erythema
-
Occasionally, nausea, headache, or dizziness
-
Long-term animal models may show osteosarcoma incidence (not confirmed in humans)
Safety & Handling
-
Usage Restriction: Laboratory research only; not approved for therapeutic or diagnostic use
-
Handling: Use sterile techniques and PPE (gloves, lab coat, goggles) during administration
-
Storage: Maintain 2–8?°C; discard after expiration
-
Disposal: Dispose per institutional biohazard protocols
Core Keywords
Teriparatide injection, Zhengu teriparatide, rhPTH 1-34 injectable, bone formation research, lab-grade osteoporosis peptide, NMPA S20240005 teriparatide, Shanghai United Cell PTH, wholesale teriparatide injection
Research Use Disclaimer
This product is exclusively for laboratory research. It is not approved for clinical, therapeutic, diagnostic, or veterinary use. Misuse may cause health or regulatory issues. Always adhere to institutional biosafety and handling protocols.
其他信息
| 重量 | 1 公斤 |
|---|---|
| 尺寸 | 26 × 23 × 26 厘米 |









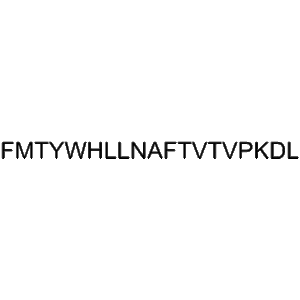
评价
目前还没有评价