描述
Product Description
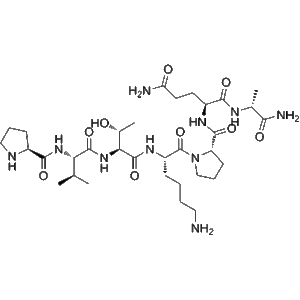
Tifuvirtide (CAS 251562-00-2), also known as T-1249, is a synthetic HIV-1 fusion inhibitor peptide developed to block viral entry and prevent infection at the earliest stage of the HIV replication cycle. Tifuvirtide represents a hybrid retroviral envelope polypeptide that combines multiple heptad repeat regions of the HIV-1 gp41 protein, enabling high-affinity binding to viral fusion intermediates. This peptide-based design allows it to effectively inhibit the formation of the six-helix bundle required for membrane fusion between the virus and host cell.
Background and Significance
HIV-1 infection is initiated when the viral envelope glycoprotein gp120 binds to the host cell CD4 receptor, followed by co-receptor engagement (CCR5 or CXCR4). This triggers conformational changes in gp41 that facilitate viral-host membrane fusion. Fusion inhibitors, such as Tifuvirtide, target gp41 to prevent this critical step, stopping viral entry before reverse transcription and integration can occur.
The significance of Tifuvirtide in research lies in its ability to:
-
Provide a precise model for studying HIV-1 viral entry mechanisms.
-
Serve as a tool for screening antiretroviral compounds targeting early infection steps.
-
Aid in understanding resistance mechanisms arising from mutations in gp41.
Unlike nucleoside reverse transcriptase inhibitors (NRTIs) or protease inhibitors, fusion inhibitors like Tifuvirtide act extracellularly and offer complementary approaches for studying combination therapies in preclinical models.
Chemical Properties and Research Utility
-
Compound Class: Synthetic retroviral envelope-derived peptide
-
Target: HIV-1 gp41 fusion domain
-
Mechanism: Blocks six-helix bundle formation, preventing membrane fusion
-
Activity: Antiretroviral, inhibits viral entry
-
Stability: Lyophilized powder ensures extended shelf life for laboratory use
-
Applications: HIV entry studies, drug resistance modeling, peptide antiviral research
Tifuvirtide’s peptide design allows high-affinity binding to fusion intermediates, minimizing off-target effects and making it a reliable tool for in vitro and in vivo preclinical studies. Its activity is validated across diverse HIV-1 subtypes, facilitating broad application in research settings.
Product Specifications
| Parameter |
Details |
| Product Name |
Tifuvirtide |
| Synonyms |
T-1249, HIV-1 fusion inhibitor peptide |
| CAS Number |
251562-00-2 |
| Molecular Formula |
Synthetic peptide (sequence proprietary) |
| Molecular Weight |
Approximate, peptide-based |
| Appearance |
White to off-white lyophilized powder |
| Purity |
≥98% (HPLC) |
| Solubility |
Soluble in water, PBS, DMSO |
| Stability |
Stable ≥24 months at –20°C |
| Storage Conditions |
Store at –20°C; avoid repeated freeze-thaw cycles |
| GMP Compliance |
Produced under GMP-certified manufacturing facility |
| Applications |
HIV-1 fusion inhibition, antiretroviral research, viral entry studies |
| Availability |
Wholesale & retail |
| Experimental Models |
In vitro cell-based HIV infection assays, viral entry models, preclinical research |
| Safety Considerations |
For laboratory research use only; not for human or veterinary use |
The detailed specifications ensure high reproducibility, structural integrity, and consistent biological activity, making Tifuvirtide suitable for extensive HIV research, including combination therapy evaluation, drug resistance studies, and mechanistic investigations.
Mechanism of Action & Research Applications
Mechanism of Action
Tifuvirtide is a potent HIV-1 gp41 fusion inhibitor. It functions through several well-characterized mechanisms:
-
Binding to gp41 Heptad Repeats: Tifuvirtide mimics the heptad repeat regions of gp41, competitively binding to fusion intermediates.
-
Inhibition of Six-Helix Bundle Formation: By preventing the folding of gp41 into the six-helix bundle, Tifuvirtide blocks viral-host membrane fusion.
-
Prevention of Viral Entry: The blockade stops HIV-1 from entering target CD4+ T cells and macrophages, halting viral replication at the earliest stage.
-
Antiretroviral Effect: By preventing infection, it reduces viral load in preclinical models and provides a tool for testing combination strategies.
Tifuvirtide’s mechanism allows researchers to study fusion inhibition independent of reverse transcription or protease activity, making it ideal for dissecting early viral entry processes.
Research Applications
1. HIV-1 Viral Entry Studies
Tifuvirtide is used to investigate HIV-1 entry pathways in various cell lines and primary immune cells. It helps delineate gp41 conformational dynamics and co-receptor interactions.
2. Antiviral Drug Screening
As a validated fusion inhibitor, Tifuvirtide serves as a positive control in high-throughput screening for new antiviral compounds targeting viral entry.
3. Resistance Mechanism Research
In vitro experiments using Tifuvirtide allow researchers to model gp41 mutations that confer resistance to fusion inhibitors, providing insight into potential clinical challenges.
4. Peptide-Based Therapeutic Development
Tifuvirtide acts as a model for designing next-generation peptide antivirals with improved stability, potency, and subtype coverage.
5. Preclinical Combination Therapy Studies
Tifuvirtide can be combined with reverse transcriptase inhibitors, protease inhibitors, or integrase inhibitors to evaluate synergistic effects in vitro, supporting translational research for combination therapies.
6. Mechanistic Studies of Viral Fusion
Researchers can use Tifuvirtide to examine the molecular steps of membrane fusion, including intermediate conformations, energy barriers, and kinetics of gp41-mediated fusion.
7. Translational Research Potential
Insights from Tifuvirtide research support the rational design of antiviral peptides and inform strategies for HIV prophylaxis and therapeutic intervention.
8. Structural Biology and Biophysics
Tifuvirtide is used in NMR, crystallography, and molecular dynamics studies to probe gp41 structure-function relationships and peptide binding modes.
9. In Vivo Preclinical Models
In animal models, Tifuvirtide can be applied to study pharmacokinetics, tissue distribution, and early-stage viral inhibition, contributing to drug development pipelines.
10. Comparative Studies with Other Fusion Inhibitors
Allows side-by-side evaluation of Tifuvirtide against other fusion inhibitors (e.g., Enfuvirtide) to assess potency, stability, and efficacy.
The versatility of Tifuvirtide in in vitro and in vivo studies, combined with GMP-grade reproducibility, makes it an essential tool for HIV research laboratories worldwide.

Side Effects (For Reference in Research Models)
Experimental observations with Tifuvirtide in preclinical and in vitro studies include:
-
Minimal Cytotoxicity: Effective concentrations generally show low cytotoxic effects on target cell lines.
-
Peptide Stability Considerations: Repeated freeze-thaw cycles may reduce activity; proper storage at –20°C is recommended.
-
Resistance Mutations: Extended in vitro exposure can lead to gp41 escape variants. Researchers should monitor for viral adaptation.
-
Species-Specific Effects: Activity and toxicity profiles may vary depending on the experimental model (rodent, primate, or cell culture).
-
Transient Cellular Responses: Some immune cells may exhibit temporary stress or metabolic changes during high-concentration exposure.
-
Pharmacodynamic Observations: Effective fusion inhibition occurs without significantly affecting non-target cellular pathways.
-
Synergistic or Antagonistic Interactions: May interact with other antiviral compounds; combination studies are recommended to characterize effects.
These effects are strictly for laboratory research reference. Tifuvirtide is not intended for human or veterinary use.
Disclaimer
For laboratory research use only. Not for human or veterinary use.
Keywords
-
Tifuvirtide CAS 251562-00-2
-
T-1249 HIV fusion inhibitor peptide
-
GMP-grade HIV research peptide
-
Synthetic antiretroviral peptide
-
HIV-1 viral entry inhibitor
-
Laboratory HIV-1 peptide compound
-
Peptide-based HIV therapeutic research
-
Fusion inhibitor for HIV preclinical studies
-
bulk supplier
-
Research use only HIV-1 peptide

 GLP-1/GIP/glucagon combination therapy The Future of Metabolic Treatment (Wholesale Supplier Guide)
2 × $2.00
GLP-1/GIP/glucagon combination therapy The Future of Metabolic Treatment (Wholesale Supplier Guide)
2 × $2.00  Tirzepatide Raw Material Factory: Low-Price Wholesale for High-Purity Peptides
1 × $1.00
Tirzepatide Raw Material Factory: Low-Price Wholesale for High-Purity Peptides
1 × $1.00  Cosmetic Peptide Raw Material Factory – Low Price Wholesale Direct from China
10 × $1.00
Cosmetic Peptide Raw Material Factory – Low Price Wholesale Direct from China
10 × $1.00  Best-Selling Weight Loss Peptide: Slimming Peptide Reta with Safe Delivery
10 × $1.00
Best-Selling Weight Loss Peptide: Slimming Peptide Reta with Safe Delivery
10 × $1.00  Wholesales Weight Loss Peptides Raw Powder CAS 129954-34-3 with Safe Delivery
1 × $2.00
Wholesales Weight Loss Peptides Raw Powder CAS 129954-34-3 with Safe Delivery
1 × $2.00 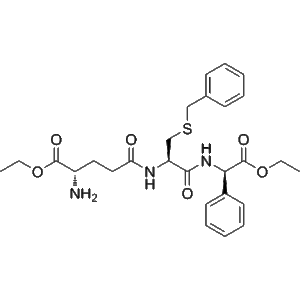 Ezatiostat (CAS: 902135-91-5) – Purity 99.71% | GMP Factory
1 × $2.00
Ezatiostat (CAS: 902135-91-5) – Purity 99.71% | GMP Factory
1 × $2.00 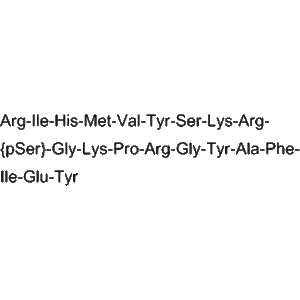 Forigerimod (IPP-201101) – CD4 T-cell Modulator Peptide for Laboratory Research
1 × $16.00
Forigerimod (IPP-201101) – CD4 T-cell Modulator Peptide for Laboratory Research
1 × $16.00 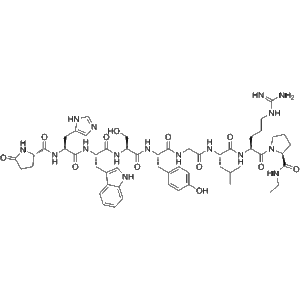 Fertirelin (CAS 38234-21-8) – GMP-Manufacturer
1 × $2.00
Fertirelin (CAS 38234-21-8) – GMP-Manufacturer
1 × $2.00 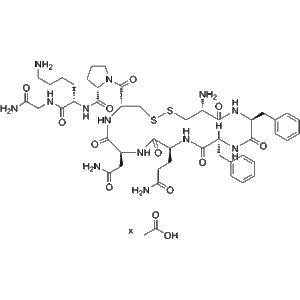 Felypressin Acetate (CAS 914453-97-7) – GMP Manufacturer
1 × $2.00
Felypressin Acetate (CAS 914453-97-7) – GMP Manufacturer
1 × $2.00 









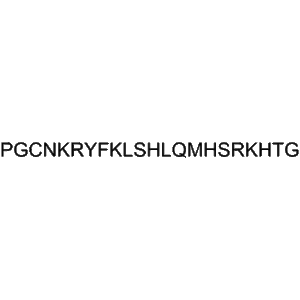
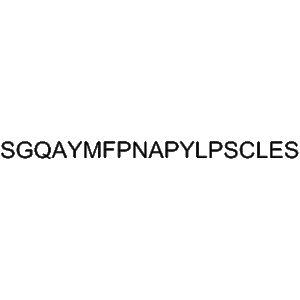
评价
目前还没有评价