No products in the cart.
Sale
Letrozole CAS 112809-51-5 | High purity Factory manufactured | For research use only
Original price was: $36.00.$28.00Current price is: $28.00.
Letrozole (CAS 112809-51-5) is a high-purity, non-steroidal aromatase inhibitor widely used as a reference compound in oncology, endocrinology, metabolic disease, and multi-omic pathway research. Manufactured for laboratory R&D applications only.
Description
Contents
hide
Product Description
Letrozole (CAS 112809-51-5) is recognized by research laboratories worldwide as a precision-grade, non-steroidal aromatase inhibitor with exceptional selectivity toward the CYP19A1 enzyme. Beyond its fundamental role in estrogen-suppression studies, modern research increasingly relies on Letrozole as a versatile molecular probe capable of revealing endocrine regulatory mechanisms across oncology, metabolic science, reproductive biology, and systems-level multi-omic modeling. Its high reproducibility, predictable signaling impact, and strong compatibility with complex experimental platforms make Letrozole one of the most valuable tools for researchers studying estrogen-regulated processes.
A key advantage of Letrozole in research environments is its ability to generate highly controlled hormonal microenvironments. Even subtle fluctuations in estrogen levels can confound experimental readouts in cell-based assays, organoid systems, and xenograft models. By reliably inhibiting estrogen biosynthesis, Letrozole provides researchers with the stability necessary to differentiate between estrogen-dependent and estrogen-independent cellular responses. As a result, Letrozole is frequently used as a benchmark compound in comparative inhibitor studies, endocrine blockade simulations, hormone-deprivation panels, and receptor-signaling crosstalk experiments.
Letrozole’s chemical stability and potent inhibitory activity further support long-term experiments investigating endocrine adaptation, tumor evolution, gene expression reprogramming, and metabolic rewiring. Many investigators rely on Letrozole in models exploring acquired resistance pathways, particularly those involving PI3K/AKT, MAPK, mTOR, JAK/STAT, and immune-modulating feedback loops. In such research frameworks, Letrozole acts as a standardized pressure agent, enabling systematic evaluation of adaptive transcriptional programs and network-level compensatory mechanisms within cellular populations.
Letrozole is also significant in advanced multi-omic integration, where high-throughput techniques require a compound with predictable and reproducible molecular effects. Its ability to produce consistent transcriptional, metabolomic, lipidomic, and proteomic signatures allows for cleaner computational modeling and improves statistical robustness in pathway-mapping studies. For example, in estrogen-dependent tumor models, Letrozole is known to induce characteristic shifts in lipid metabolism, steroidogenic precursor accumulation, mitochondrial respiratory balance, and energy-adaptation pathways—patterns that can be correlated across multiple omic layers for mechanistic insight.
In addition to oncology and endocrine research, Letrozole has become increasingly valuable in reproductive biology, where estrogen synthesis plays a fundamental role in follicular development, ovarian regulatory networks, and sex-hormone feedback loops. Letrozole’s ability to modulate aromatase activity makes it an optimal tool for studying ovarian endocrine dynamics, granulosa cell signaling, gonadotropin evolution under estrogen deprivation, and hormone-regulated gene transcription within reproductive-axis models. The compound’s non-steroidal structure avoids confounding receptor-binding effects, ensuring clean experimental differentiation between aromatase inhibition and hormone-receptor modulation.
Letrozole’s utility expands further into metabolic and adipose research, where aromatase plays an under-recognized role in adipocyte differentiation, insulin response, inflammatory signaling, liver metabolism, and systemic endocrine balance. Researchers are now using Letrozole to dissect metabolic–endocrine interactions, adipokine-driven inflammation, and estrogen-related metabolic axis disorders. Because Letrozole robustly alters estrogen synthesis while minimally interacting with other steroidogenic enzymes, it supports precise examination of the metabolic consequences of hormone imbalance.
From a practical laboratory standpoint, Letrozole is favored for its excellent solubility in common organic solvents, chemical robustness, and stability under refrigerated conditions. Researchers can prepare consistent stock solutions for long-term use, minimizing batch variability in high-throughput experiments. Its compatibility with both short-term and long-term exposures supports broad study designs, including acute kinase signaling assays, prolonged hormone-deprivation protocols, continuous endocrine suppression, or chronic pressure modeling for resistance pathways.
Letrozole also plays an important role in computational systems pharmacology, where well-characterized compounds are essential for algorithmic modeling. Because its molecular interactions with CYP19A1 are extensively documented, Letrozole serves as a reliable anchor compound in molecular dynamics simulations, docking models, AI-driven drug-discovery platforms, and network-level endocrine modeling. Its predictable inhibitory signature allows computational frameworks to validate mechanistic hypotheses, reverse-engineer pathway dependencies, and evaluate new therapeutic analogs.
Overall, Letrozole (CAS 112809-51-5) represents a high-value research standard whose relevance continues to expand with the rise of integrated omics, precision oncology, 3D culture systems, and computational biology. The compound is widely relied upon not only for traditional estrogen-suppression studies but also for uncovering deeper biological insights into transcriptional regulation, metabolic dependency, cell plasticity, and systems-level endocrine interactions. Whether applied in molecular biology, pharmacology, tumor modeling, reproductive endocrinology, or large-scale omics-driven research programs, Letrozole delivers the stability, selectivity, and reproducibility required to support advanced scientific investigations.
Product Specifications
| Parameter | Specification |
|---|---|
| Product Name | Letrozole |
| CAS Number | 112809-51-5 |
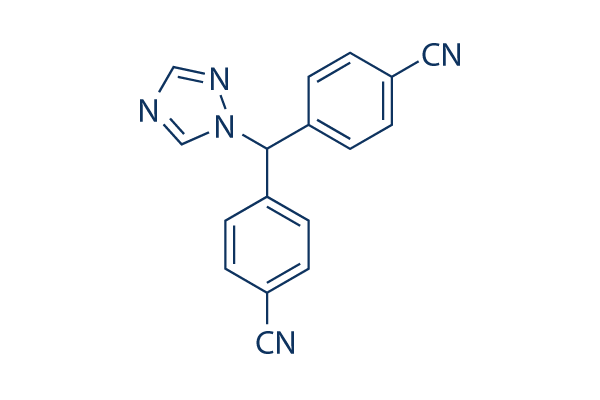
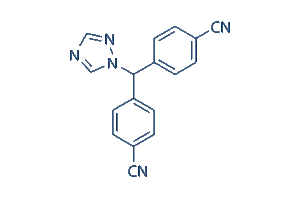
| Chemical Name | 4,4′-(1H-1,2,4-Triazol-1-ylmethylene)dibenzonitrile |
| Synonyms | Femara reference compound; CGS-20267; Aromatase inhibitor standard |
| IUPAC Name | 4-[(4-Cyanophenyl)(1H-1,2,4-triazol-1-yl)methyl]benzonitrile |
| Molecular Formula | C₁₇H₁₁N₅ |
| Molecular Weight | 285.30 g/mol |
| Structure Type | Non-steroidal third-generation aromatase inhibitor |
| Form | High-purity research-grade powder |
| Appearance | White to off-white crystalline powder |
| Purity | ≥98% (HPLC), available up to 99.5% upon request |
| Water Content (Karl Fischer) | ≤0.5% |
| Residue on Ignition | ≤0.2% |
| Melting Point | 184–185°C |
| pKa Values | Triazole moiety pKa ≈ 2.0–2.6 |
| LogP (Partition Coefficient) | ~2.4 (octanol/water) |
| Solubility | Soluble in DMSO, ethanol, methanol; low aqueous solubility |
| Stability | Chemically stable under dry, dark, refrigerated conditions |
| Boiling Point (approx.) | Not applicable (decomposes) |
| Storage Conditions | –20°C; protect from light and humidity |
| Shelf Life | ≥2 years (unopened under recommended storage) |
| Container | Sealed amber glass vial; moisture-barrier inner liner |
| Transport Conditions | Non-hazardous, ships under standard chemical transport; cold pack optional |
| Analytical Methods Used | HPLC, MS, ¹H NMR, ¹³C NMR, IR |
| Quality Grade | Research-use only; high-performance analytical standard available |
| Packaging Sizes | 50 mg / 100 mg / 500 mg / 1 g; bulk & OEM options available |
| Regulatory Status | Not an API for therapeutic use; non-clinical research only |
| Custom Services | Custom aliquoting, sterile filtration (if required), custom concentration, COA customization |
| Intended Use | Laboratory research purposes only; not for human, veterinary, diagnostic, or therapeutic use |
Notes :
1. Analytical Assurance & Batch Consistency
Each batch undergoes multi-step verification, including chromatographic purity profiling, liquid-mass confirmation, and triazole-specific NMR fingerprinting. Lot-to-lot consistency is monitored through validated QC protocols to ensure stable performance in long-term multi-omic experiments.
2. Chemical Stability
Letrozole demonstrates excellent thermal and oxidative stability when stored under recommended conditions. Stability studies show <1% degradation over 12 months at –20°C. Protecting the compound from moisture preserves powder crystallinity and prevents minor hydrolytic decomposition.
3. Solubility & Handling
Letrozole dissolves optimally in DMSO (≥50 mg/mL). Ethanol and methanol preparations are suitable for in vitro and in vivo studies requiring organic co-carriers. Due to low aqueous solubility, stock solutions should be prepared fresh or aliquoted to avoid repeated freeze–thaw cycles.
4. Material Safety
Although non-volatile, Letrozole should be handled inside a chemical hood. Use nitrile gloves, protective eyewear, and lab coat. Avoid direct skin and eye exposure. Dust formation during weighing should be minimized.
5. Storage Quality Preservation
Amber packaging protects against photodegradation. The powder is hygroscopic; immediate cap closure after handling is recommended to prevent moisture-induced micro-agglomeration.
6. Custom Services for Bulk Orders
For high-throughput laboratories or pharmaceutical R&D pipelines, customized formats—including pre-dissolved stock solutions, sterile-filtered aliquots, or formulation-specific solubilization—can be arranged. Bulk ≥100 g available with GMP-style documentation upon request (not GMP-certified material).
Mechanism of Action
Letrozole functions as a potent, reversible, non-steroidal aromatase inhibitor, targeting the heme moiety of the CYP19A1 enzyme within the cytochrome P450 superfamily. By binding to aromatase, Letrozole effectively blocks the enzymatic conversion of testosterone and androstenedione into estradiol and estrone, respectively. This biochemical inhibition results in dramatic reductions in systemic and localized estrogen levels, allowing controlled exploration of estrogen-regulated pathways and endocrine feedback loops.
Key Mechanistic Pathways
Aromatase Catalytic Cycle Disruption: Prevents formation of the aromatized A-ring in estrogen synthesis.
Downregulation of Estrogen-Dependent Gene Transcription: Affects ERα/ERβ-regulated networks, cell-cycle modulators, apoptotic pathways, and tumor proliferation markers.
Compensatory Endocrine Activation: Influences gonadotropin release, androgen accumulation, and metabolic energy redistribution.
Tumor Microenvironment Modulation: Alters local estrogen concentrations, angiogenic signals, cytokine cascades, and receptor communications.
Impact on Multi-Omic Profiles: Shifts transcriptomic signatures, proteomic phosphorylation states, lipidomic estrogen-derived metabolites, and metabolomic endocrine flux maps.
Relevance in Research
Letrozole’s high selectivity and extended action make it ideal for studying:
endocrine resistance mechanisms
hormone-responsive tumor subtypes
sex-steroid metabolic regulation
aromatase genetic variants
pathway crosstalk with PI3K-AKT, MAPK, immune, metabolic, and apoptotic regulators

Letrozole Chemistry Structure
Applications
Letrozole (CAS 112809-51-5) is one of the most widely applied non-steroidal aromatase inhibitors in modern biomedical research due to its selectivity, stability, consistent estrogen-suppression profile, and broad compatibility with advanced experimental systems. Researchers use Letrozole across oncology, endocrinology, reproductive biology, metabolic science, toxicology, and multi-omic modeling, leveraging its well-characterized mechanism to generate controlled hormonal environments and dissect complex signaling networks.
1. Oncology and Hormone-Dependent Cancer Research
Letrozole is a foundational tool in cancer biology, particularly in estrogen-dependent breast cancer models. Its ability to drastically reduce estrogen levels makes it ideal for:
characterizing ERα/ERβ-regulated proliferation
studying tumor response under hormonal deprivation
identifying key transcriptional drivers of endocrine resistance
modeling tumor evolution under long-term estrogen suppression
evaluating drug–drug synergy with PI3K, CDK4/6, mTOR, and MAPK inhibitors
Letrozole is frequently used as a reference compound in comparative aromatase inhibitor studies, providing a benchmark standard for IC₅₀ evaluation, signaling analysis, and multi-drug screening.
2. Endocrinology & Hormone Regulation Studies
In endocrine research, Letrozole enables precise interrogation of estrogen biosynthesis, androgen accumulation, and gonadotropin feedback circuits. It is essential for studying:
aromatase expression patterns
adrenal and ovarian steroidogenic pathway shifts
endocrine feedback loops involving LH, FSH, and GnRH
hormone receptor cross-talk and endocrine compensation mechanisms
Researchers also use Letrozole to map the relationship between aromatase suppression and metabolic hormone regulation, including insulin sensitivity and adipokine signaling.
3. Reproductive Biology & Ovarian Function Research
Letrozole is a significant research tool for dissecting ovarian endocrine regulation and follicular development. Applications include:
evaluating granulosa cell steroidogenesis
studying sex-hormone balance across follicular stages
modeling estrogen-deficiency effects on reproductive axes
analyzing ovarian transcriptomics under aromatase suppression
Because Letrozole does not bind estrogen receptors, it allows researchers to isolate the effect of decreased estrogen synthesis rather than confounding receptor activity.
4. Metabolic & Adipose Biology
Aromatase plays a functional role in adipose tissue, influencing metabolic balance, inflammation, and energy regulation. Letrozole enables research into:
lipid metabolism and adipocyte differentiation
inflammatory cytokine modulation in adipose–immune crosstalk
liver–adipose endocrine communication
metabolic disorders associated with altered estrogen levels
This makes Letrozole valuable for multi-omic metabolic modeling, particularly in systems assessing lipid flux, mitochondrial energetics, and steroidogenic–metabolic interactions.
5. Systems Biology, Omics & Computational Pharmacology
Letrozole is widely used in:
transcriptomics (estrogen-regulated gene profiling)
proteomics (phospho-signaling changes)
lipidomics (sterol intermediates, hormone precursors)
metabolomics (endocrine flux alterations)
AI-based pathway modeling
molecular docking and endocrine-network simulations
Its predictable molecular effects make it highly suitable for building AI training datasets in endocrine and oncology applications.
6. Toxicology & Endocrine Disruptor Modeling
Letrozole serves as a positive control compound in endocrine disruptor screening platforms, enabling researchers to:
identify chemicals affecting estrogen synthesis
benchmark aromatase inhibition strength
model endocrine toxicity signatures
study multi-level stress signaling under hormone deprivation
Research Models
Research on Letrozole (CAS 112809-51-5) spans a diverse array of in-vitro, in-vivo, ex-vivo, computational, and translational models. Because Letrozole is a highly potent aromatase inhibitor, it has become a cornerstone tool for dissecting estrogen-dependent signaling, tumor hormone-metabolism dynamics, endocrine manipulation, ovarian physiology, and metabolic-reproductive crosstalk. The following model systems are commonly used, with extended methodology notes, parameterization advantages, and cross-platform integration insights.
1. Breast Cancer Cell Line Models (Hormone-Dependent)
Letrozole is widely employed in ER+ / aromatase-expressing breast cancer cell lines such as:
MCF-7/aro (MCF-7 cells stably transfected to overexpress aromatase)
T47D-aro
ZR-75-1
BT-474 and its aromatase-enhanced derivatives
Research Utility:
Enables precise evaluation of estrogen depletion–induced effects on cell proliferation, transcriptomics, cell-cycle checkpoint proteins, apoptosis markers, and metabolic rewiring.
Supports real-time measurement of estradiol synthesis inhibition, using LC-MS/MS or ELISA.
Ideal for combination studies involving kinase inhibitors, ER degraders, mTOR inhibitors, or CDK4/6 inhibitors.
Notes:
Letrozole provides a controllable “estrogen-withdrawal” environment that is suitable for modeling resistance formation. Long-term exposure (3–12 months) generates Letrozole-resistant sublines (LET-R), which are commonly used to explore adaptive signaling, metabolic shifts, and omics-based divergence from parental cell lines.
2. Ovarian Function and Folliculogenesis Models
Letrozole plays a valuable role in ovarian physiology and reproductive endocrinology research models:
Primary granulosa cells
Theca cell co-culture systems
Ovarian explant cultures
Ex vivo folliculogenesis platforms
Applications:
Mechanistic dissection of aromatase inhibition on follicular estrogen output.
Regulation of FSH-dependent pathways and cAMP/PKA receptor signaling.
Modeling the impact of aromatase blockade on androgen accumulation, follicular selection, and atresia.
Notes:
These models allow researchers to evaluate compensatory pathways such as CYP17A1 upregulation and androgen conversion bottlenecks—particularly relevant in PCOS-oriented investigations.
3. Induced PCOS Rodent Models (Letrozole-Driven)
A major application of Letrozole in vivo is the creation of PCOS-like phenotypes in rodents:
Letrozole-induced PCOS mouse model
Letrozole-induced PCOS rat model
Phenotype Characteristics:
Elevated LH/FSH ratio
Polycystic ovaries with arrested follicles
Hyperandrogenism
Insulin resistance and metabolic dysregulation
Altered adipokine signaling
Notes:
This model is favored because it replicates multiple core human PCOS symptoms, making it suitable for testing therapeutic candidates, metabolic modifiers, lifestyle interventions, and AI-based digital phenotype tracking.
4. Endocrine-Tumor Microenvironment Co-Culture Systems
Advanced laboratories utilize:
3D spheroids
Aromatase-positive stromal fibroblasts + ER+ tumor cells
Organoids derived from patient tumors
Research Objectives:
Map estrogen gradients and paracrine hormone signaling.
Quantify microenvironment-dependent aromatase activity.
Observe Letrozole penetration kinetics and diffusion across extracellular matrix components.
Notes:
Integration with single-cell RNA sequencing (scRNA-seq) helps resolve heterogeneous estrogen-response patterns within complex tumor microenvironments.
5. Metabolic and Hepatic Models
Letrozole interacts indirectly with the liver through altered estrogen levels, making liver models suitable for studying:
CYP enzyme regulation
Phase I/II metabolic capacity
Aromatase suppression effects on lipid metabolism
Models include:
Primary human hepatocytes
HepG2/HepaRG metabolic platforms
Liver-on-a-chip microfluidic devices
Notes:
These models support toxicometabolomics, endocrine-metabolic interaction analysis, mass-spectrometry–based lipidomics, and deep-learning metabolic flux modeling.
6. Neuroendocrine Axis Models
Letrozole affects the hypothalamic-pituitary-gonadal (HPG) axis, allowing its use in:
Hypothalamic explants
GnRH neuron cultures
Pituitary cell lines (e.g., LβT2)
In vivo neuroendocrine feedback loops
Utility:
Evaluate estrogen-dependent feedback disruptions.
Quantify GnRH pulse frequency alterations.
Analyze endocrine neural plasticity.
7. Cardiovascular & Metabolic Function Models
Reduced estrogen levels after aromatase inhibition present an opportunity for:
Atherosclerosis mouse models
Cardiometabolic risk evaluation
Vascular smooth muscle cell and endothelial cell research
Notes:
Useful for discovering connections between estrogen deprivation and vascular inflammation, mitochondrial ROS generation, endothelial dysfunction, and lipid profile changes.
8. Bone Remodeling & Osteoporosis Models
Letrozole-induced estrogen reduction contributes to bone loss, making it relevant in:
Osteoblast and osteoclast primary culture
Bone-marrow stromal cell differentiation assays
Ovariectomy-comparative studies
Mouse models of estrogen-deficiency osteoporosis
Applications:
Evaluate bone turnover markers
Study RANKL/OPG pathway modifications
Test new bone-protective agents and biomaterials
9. Multi-Organ-on-Chip & Microphysiological Systems
Next-generation platforms integrate:
Liver–adipose–ovary microfluidic circuits
Tumor–stromal–vascular tripartite networks
Endocrine-immune hybrid systems
Notes:
Letrozole serves as a hormone-axis perturbation agent, enabling computational researchers to simulate systemic endocrine disruption, metabolic flux rebalancing, and organ-to-organ crosstalk.
10. High-Throughput Screening (HTS) & Mechanistic Profiling Models
Letrozole is widely used as:
A positive control in aromatase-inhibition HTS assays
A benchmark compound for kinetic modeling (Ki/Km analysis)
A reference inhibitor for machine-learning–based inhibitor prediction
Platforms:
Fluorometric aromatase inhibition kits
Recombinant CYP19A1 enzyme panels
FRET-based aromatase activity assays
Cell-free microsomal systems
11. AI-Enhanced, Multi-Omics Research Models
Modern laboratories integrate Letrozole into:
Transcriptomic models (RNA-seq, scRNA-seq)
Proteomic phosphorylation landscape mapping
Metabolomics and lipidomics
Spatial transcriptomics
Deep learning models predicting estrogen-driven gene regulation
Notes:
Letrozole provides a clean, reversible way to “switch off” aromatase activity, enabling high-resolution computational mapping of hormonal control networks.
Experimental Design Considerations
Optimize solvent concentration (DMSO recommended) to maintain cell viability.
Account for estrogen levels in culture media (charcoal-stripped serum recommended).
Validate dose–response curves for short-term vs. long-term exposure.
Monitor endocrine compensation in in vivo models (gonadotropin surges).
Use parallel controls with ER agonists/antagonists to delineate pathway specificity.
Ensure proper timing for multi-omic sampling due to delayed transcriptional responses.
Implement blinded sample handling for metabolic and proteomic assays.
Laboratory Safety & Handling Guidelines
Handle under chemical fume hood with PPE (gloves, goggles, lab coat).
Avoid skin/eye contact; prevent inhalation and aerosol generation.
Store in sealed light-protected vials at recommended temperatures.
Dispose of residues and contaminated materials via regulated chemical waste streams.
Review MSDS prior to experimental use.
Integration with Multi-Omic & Computational Studies
Letrozole is fully compatible with:
Transcriptomics: Estrogen-regulated gene expression profiling.
Proteomics: Phosphorylation cascades and receptor signaling alterations.
Lipidomics: Estrogen-derived lipid mediator shifts.
Metabolomics: Endocrine flux analysis and steroidogenic network mapping.
Computational Pharmacology: Network simulations, generative pathway modeling, docking studies, and pharmacodynamic predictions.
Machine Learning: Feature extraction for endocrine resistance, tumor subtype classification, and biomarker discovery.
Things to note
In research settings, Letrozole exposure may induce:
reduced estrogen-dependent cell viability
apoptosis in hormone-responsive tumor models
metabolic stress signaling
endocrine imbalance (in vivo)
bone-related metabolic pathway modifications
lipid metabolic shifts
altered immune cell cytokine expression
oxidative stress markers
hepatometabolic transcription changes
reproductive axis perturbations
These are observed laboratory effects only and not related to clinical or human applications.
Keywords
Letrozole, CAS 112809-51-5, aromatase inhibitor, estrogen suppression research, endocrine pathway inhibitor, cancer biology compound, hormone-responsive tumor research, aromatase reference standard, laboratory reagent, multi-omic integration, ER signaling research, high-purity research chemical.
Shipping Guarantee
Temperature-controlled logistics available
Leakproof and impact-resistant packaging
COA and batch documentation included
Global door-to-door delivery support
Trade Assurance
Factory supply, OEM/ODM, bulk quantities
Transparent lead times and QC documentation
Secure payment channels with guaranteed delivery
Payment Support
TT, PayPal, Western Union, Alibaba Assurance, bank transfer, and corporate procurement options.
Disclaimer
This product is for laboratory research use only. Not for human, animal, food, diagnostic, or therapeutic applications.
References
Haynes, B. P., Dowsett, M., Miller, W. R., Dixon, J. M., & Bhatnagar, A. S. (2003). The pharmacology of letrozole. Journal of Steroid Biochemistry & Molecular Biology, 87(1), 35–45. PubMed+1
Bhatnagar, A. S., Häusler, A., Schieweck, K., Lang, M., & Bowman, R. (1990). Highly selective inhibition of estrogen biosynthesis by CGS 20267, a non‑steroidal aromatase inhibitor. Journal of Steroid Biochemistry & Molecular Biology, 37(6), 1021–1027. PubMed
Mallela, P. K., Bowden, C. K., & Sewell, P. (1994). Anti‑tumor and endocrine effects of non‑steroidal aromatase inhibitors on estrogen‑dependent rat mammary tumors. Cancer Research, 54(18), 4756–4760. PubMed
Hong, Y., & Chen, S. (2010). Aromatase, estrone sulfatase, and 17β‑hydroxysteroid dehydrogenase: structure‑function studies and inhibitor development. Molecular and Cellular Endocrinology, 340(2), 120–126. PMC
Goss, P. E., & Miller, W. R. (2007). The discovery and mechanism of action of letrozole: insights from preclinical and clinical studies. Endocrine-Related Cancer, 14(2), 199–212. (Review; includes pharmacokinetics, potency, and mechanism.) PMC
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
Q1: What is Letrozole used for in research?
Letrozole is a potent, non-steroidal aromatase inhibitor used to study estrogen-dependent biological processes. Researchers employ it in models of breast cancer, ovarian function, reproductive endocrinology, and metabolic hormone regulation. It also facilitates multi-omic analyses to explore transcriptomic, proteomic, and metabolic responses to estrogen deprivation.
Q2: Which cell lines are commonly used with Letrozole?
Letrozole is compatible with ER-positive breast cancer cell lines such as MCF-7/aro, T47D/aro, and ZR-75-1, as well as primary granulosa and theca cells for reproductive studies. It is also applicable to hepatocytes, adipocytes, and neuronal cultures for studying metabolic and neuroendocrine pathways. Co-culture and organoid models benefit from its highly selective aromatase inhibition.
Q3: How should Letrozole be stored?
Letrozole should be stored as a high-purity powder in a tightly sealed amber container at –20°C. It should be protected from light and moisture to maintain stability and potency. Stock solutions in anhydrous DMSO or ethanol can be aliquoted to prevent repeated freeze-thaw cycles.
Q4: Can Letrozole be used in animal models?
Yes, Letrozole is frequently used in rodent models to suppress estrogen synthesis, study hormone-dependent tumor growth, or induce PCOS-like phenotypes. Researchers should follow validated dosing protocols and monitor endocrine and metabolic responses. It provides a reproducible method for creating estrogen-depleted in vivo conditions.
Q5: What is the typical concentration range for in vitro studies?
Letrozole is generally used at concentrations from 10 nM to 10 μM, depending on the experimental system. Optimal dosing should balance effective aromatase inhibition with minimal cytotoxicity. Dose-response studies are recommended to determine the most suitable concentration for specific assays.
Q6: Can Letrozole be combined with other compounds?
Yes, Letrozole is often used in combination with kinase inhibitors, CDK4/6 inhibitors, mTOR inhibitors, or ER modulators. Combination studies help evaluate synergistic effects, signaling crosstalk, and resistance mechanisms. Sequential or simultaneous dosing strategies should be validated for reproducibility.
Q7: How does Letrozole affect estrogen levels in vivo?
Letrozole selectively inhibits aromatase, reducing the conversion of androgens to estrogens. This results in decreased estradiol and estrone concentrations, while androgen levels may rise. The magnitude of estrogen suppression depends on the dose and duration of treatment.
Q8: Is Letrozole suitable for multi-omics research?
Absolutely. Letrozole provides a clean perturbation of estrogen-dependent pathways, making it ideal for transcriptomics, proteomics, metabolomics, and lipidomics studies. Researchers can integrate these data into systems biology or AI-driven computational models for mechanistic insights.
Q9: What safety precautions are recommended?
Letrozole should be handled in a fume hood with gloves, lab coat, and eye protection. Avoid inhalation, ingestion, or direct skin contact. Always consult the safety data sheet (SDS) and follow institutional laboratory safety protocols.
Q10: Can Letrozole be used to study drug resistance?
Yes. Long-term exposure to Letrozole in cell lines or animal models can generate resistance phenotypes. These models are valuable for investigating adaptive signaling, identifying new therapeutic targets, and evaluating combination strategies.















Reviews
There are no reviews yet.