No products in the cart.
Sale
Mitoxantrone Hydrochloride Injection | High purity | Factory manufactured – low price wholesale
Original price was: $6.00.$2.00Current price is: $2.00.
Mitoxantrone Hydrochloride Injection (CAS 70476-82-3) is a synthetic anthracenedione antineoplastic agent that intercalates into DNA and inhibits topoisomerase II, disrupting DNA replication and transcription. Supplied as a sterile injectable solution, it is designed for in vitro and in vivo preclinical research, including oncology, hematologic malignancies, and autoimmune disease studies.
Description
Product Description
1. Molecular Characteristics and Formulation
Mitoxantrone Hydrochloride Injection (CAS 70476-82-3) is a synthetic anthracenedione compound with potent antineoplastic activity. It functions as a DNA intercalator and topoisomerase II inhibitor, inducing double-strand DNA breaks, cell cycle arrest, and apoptosis. The injectable formulation is prepared under GMP-compliant conditions, ensuring high purity, chemical stability, and reproducible pharmacological effects. Supplied as a sterile aqueous solution, it supports precise dosing in both in vitro cytotoxicity studies and in vivo preclinical models.
2. Antineoplastic Mechanism
Mitoxantrone Hydrochloride binds DNA intercalatively, stabilizing topoisomerase II-DNA cleavable complexes and preventing DNA religation. This results in accumulation of DNA strand breaks, triggering p53-dependent apoptosis, cell cycle arrest in G2/M phase, and inhibition of cell proliferation. It is widely applied to study leukemia, lymphoma, breast cancer, and prostate cancer preclinical models.
3. Immunomodulatory Applications
Beyond its cytotoxic effects, Mitoxantrone Hydrochloride exhibits immunomodulatory activity, making it suitable for research in autoimmune diseases such as multiple sclerosis (MS). It selectively depletes pathogenic T cells and B cells, modulates cytokine expression, and preserves regulatory immune populations, providing a mechanistic tool for studying immune-mediated pathologies.
4. Metabolic and Cellular Research Applications
Mitoxantrone is used to investigate DNA damage response pathways, apoptosis mechanisms, and cell cycle regulation. It enables studies on drug resistance mechanisms, topoisomerase II dynamics, and genotoxic stress responses in diverse cancer cell lines and primary cultures. Researchers can combine it with flow cytometry, Western blotting, and transcriptomic assays to assess cellular responses to DNA damage and cytotoxic stress.
5. Pharmacological Research and Preclinical Models
Mitoxantrone Hydrochloride Injection serves as a reference standard in pharmacology, pharmacokinetics (PK), and pharmacodynamics (PD) research. It supports dose-response studies, toxicity assays, and combination therapy evaluations. Integration with animal models allows simulation of drug metabolism, tissue distribution, and therapeutic efficacy in both hematologic and solid tumor preclinical studies.
Product Specifications
| Parameter | Specification / Details |
|---|---|
| Product Name | Mitoxantrone Hydrochloride Injection |
| CAS Number | 70476-82-3 |
| Molecular Formula | C₂₂H₂₈Cl₂N₄O₆ |
| Molecular Weight | 517.4 g/mol |
| Product Type | Synthetic anthracenedione, antineoplastic agent, sterile injectable solution |
| Formulation | Sterile aqueous solution with stabilizers (e.g., sodium chloride, buffers) |
| Appearance | Clear to slightly yellow solution |
| Purity | ≥ 99% (HPLC validated) |
| Endotoxin Level | ≤ 0.1 EU/mg |
| Concentration | 2 mg/mL, 4 mg/mL, 10 mg/mL (customizable) |
| pH | 3.0–4.5 |
| Sterility | USP <71> compliant |
| Residual Solvents | Meets ICH Q3C guidelines |
| Storage Conditions | 2–8°C, protect from light |
| Shelf Life | 12–24 months under recommended storage |
| Packaging Options | 1 mL, 5 mL, 10 mL vials; bulk solution ≥100 mL |
| Customization / OEM | Concentration, vial size, and private labeling available |
| Documentation Provided | COA, MSDS, TDS, lot-specific data |
Mechanism of Action
1. DNA Intercalation
Mitoxantrone Hydrochloride functions primarily as a DNA intercalator, inserting between base pairs of double-stranded DNA. This intercalation disrupts DNA helical structure, impeding DNA replication and transcription. The resulting structural perturbations trigger DNA damage response pathways, making it a reliable tool for studying genotoxic stress and apoptotic mechanisms in preclinical research.
2. Topoisomerase II Inhibition
Mitoxantrone selectively inhibits topoisomerase II, a key enzyme that mediates DNA strand break religation during replication. By stabilizing the topoisomerase II-DNA cleavable complex, it prevents repair of DNA double-strand breaks, leading to accumulation of DNA damage. This mechanism underlies its cytotoxic effect on rapidly dividing cells and is widely applied in cancer biology research, including leukemia, lymphoma, and solid tumor models.
3. Induction of Apoptosis and Cell Cycle Arrest
DNA damage induced by Mitoxantrone activates p53-dependent and independent pathways, resulting in G2/M cell cycle arrest and programmed cell death. Researchers use it to study apoptotic signaling cascades, including caspase activation, Bcl-2 family modulation, and mitochondrial depolarization, providing mechanistic insights into chemotherapeutic cytotoxicity.
4. Immunomodulatory Effects
In addition to direct cytotoxicity, Mitoxantrone Hydrochloride Injection exhibits immunomodulatory properties. It selectively affects T cells, B cells, and other immune subsets, reducing pathogenic immune populations while sparing regulatory cells. This property is exploited in autoimmune disease models such as multiple sclerosis, enabling investigation of immune-mediated pathology, cytokine regulation, and therapeutic mechanisms.
5. Applications in DNA Damage and Drug Resistance Studies
Mitoxantrone is a critical tool for studying DNA damage response (DDR) pathways, topoisomerase II dynamics, and drug resistance mechanisms. By inducing controlled genotoxic stress, researchers can explore cellular repair mechanisms, checkpoint activation, and apoptosis thresholds. It is also used in combination therapy experiments to evaluate synergistic cytotoxic effects and potential resistance mechanisms in preclinical oncology research.
6. Experimental Pharmacology and Translational Research
The well-characterized mechanism of action makes Mitoxantrone Injection suitable for pharmacokinetic (PK) and pharmacodynamic (PD) studies, including dose-response assays, tissue distribution, and bioavailability assessments. It provides a reference standard for experimental antineoplastic agents, enabling reproducible validation of assay protocols and preclinical therapeutic strategies.
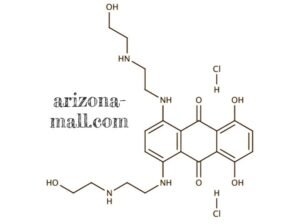
Applications
1. Preclinical Oncology Research
Mitoxantrone Hydrochloride Injection is widely used in preclinical cancer research to investigate antineoplastic mechanisms, cytotoxicity, and tumor cell apoptosis. Its ability to intercalate DNA and inhibit topoisomerase II makes it a reliable tool for studying cell cycle arrest, DNA damage response, and tumor cell proliferation in leukemia, lymphoma, breast cancer, and prostate cancer models. Researchers utilize it to evaluate dose-dependent cytotoxic effects, combinatorial therapies, and mechanisms of drug resistance.
2. Hematologic Malignancy Models
In hematologic research, Mitoxantrone is employed to study leukemia and lymphoma pathophysiology. It induces double-strand DNA breaks, leading to apoptosis in rapidly dividing hematopoietic cells. Experimental models often combine flow cytometry, cytogenetic analysis, and molecular assays to quantify cell cycle arrest, apoptosis markers, and DNA damage responses.
3. Immunomodulation and Autoimmune Disease Studies
Mitoxantrone Hydrochloride exhibits selective immunomodulatory effects, making it useful in autoimmune disease research such as multiple sclerosis (MS) and systemic lupus erythematosus (SLE). It depletes pathogenic T and B cells, modulates cytokine secretion, and preserves regulatory immune populations, enabling mechanistic studies of immune-mediated tissue damage.
4. Cell Cycle and Apoptosis Research
Researchers utilize Mitoxantrone to investigate cell cycle dynamics, apoptosis pathways, and DNA repair mechanisms. Its action induces G2/M arrest and caspase-dependent apoptosis, allowing detailed studies on checkpoint regulation, Bcl-2 family modulation, and mitochondrial pathways. This is essential for drug screening, combination therapy evaluations, and understanding chemoresistance mechanisms.
5. Pharmacological and Translational Studies
As a well-characterized antineoplastic agent, Mitoxantrone Injection serves as a reference for pharmacokinetics (PK), pharmacodynamics (PD), and dose-response studies. It supports evaluation of novel topoisomerase II inhibitors, biosimilars, and experimental anticancer compounds in both in vitro and in vivo models. Researchers can assess tissue distribution, metabolic stability, and therapeutic indices for translational research.
6. Drug Resistance and Mechanistic Investigations
Mitoxantrone is employed to study drug resistance mechanisms, particularly topoisomerase II-mediated resistance and multidrug resistance (MDR) pathways. Researchers can combine it with efflux pump inhibitors, checkpoint modulators, or DNA repair pathway inhibitors to elucidate resistance mechanisms and optimize preclinical therapeutic strategies.
7. Biomolecular and Genotoxicity Studies
Its precise action on DNA and topoisomerase II allows researchers to study DNA damage response (DDR), genomic instability, and genotoxicity assays. Mitoxantrone is commonly used with comet assays, γ-H2AX staining, and transcriptomic profiling to assess cellular responses to DNA damage and mechanistic pathways of apoptosis.
Side Effects
1. In Vitro Experimental Observations
In cell culture studies, Mitoxantrone Hydrochloride Injection is generally well-tolerated at recommended concentrations. Excessive doses may cause off-target cytotoxicity, including non-specific DNA damage and altered cell viability in non-target cell lines. Researchers should optimize concentration and exposure time to ensure specific topoisomerase II inhibition while minimizing unintended cellular effects.
2. In Vivo Animal Models
In preclinical animal studies, Mitoxantrone may induce:
Myelosuppression: reduction in white blood cells, red blood cells, and platelets
Cardiotoxicity risk: particularly with high cumulative doses or prolonged administration
Gastrointestinal effects: mild anorexia, weight loss, or diarrhea in sensitive models
Bone marrow suppression: requiring careful monitoring of hematologic parameters
These effects are dose-dependent and typically reversible in short-term studies. Researchers should design experiments to monitor hematologic indices and cardiac function markers.
3. Immunomodulatory and Inflammatory Effects
Mitoxantrone Hydrochloride can transiently modulate T cell, B cell, and macrophage populations in animal models. While useful for autoimmune disease studies, excessive dosing may alter cytokine profiles or cause unintended immune suppression, potentially confounding experimental results.
4. Metabolic and Systemic Effects
In vivo administration may influence hepatic enzyme activity and metabolic pathways, including mild alterations in liver function markers or glucose metabolism in certain models. Researchers should account for these systemic effects when interpreting metabolic or toxicity studies.
5. Experimental Precautionary Measures
To minimize side effects and experimental artifacts:
Use recommended dosing and exposure durations
Monitor hematologic parameters, liver enzymes, and cardiac markers in long-term animal studies
Handle Mitoxantrone Injection under Biosafety Level 2 (BSL-2) conditions
Avoid excessive repeated dosing in sensitive models
Store at 2–8°C, protect from light, and prevent repeated freeze-thaw cycles to maintain biological activity
Mitoxantrone Hydrochloride Injection is intended for research use only and should not be used for human or veterinary clinical applications outside controlled experimental models.
Keywords
Mitoxantrone Hydrochloride Injection, CAS 70476-82-3, anthracenedione antineoplastic agent, DNA intercalator, topoisomerase II inhibitor, cytotoxic agent, apoptosis inducer, cell cycle arrest, hematologic malignancy research, leukemia model, lymphoma model, breast cancer preclinical studies, prostate cancer research, immune modulation, autoimmune disease model, genotoxicity studies, pharmacology research, preclinical oncology, experimental antineoplastic drug, injectable solution
Shipping Guarantee
Cold-Chain Shipping: All Mitoxantrone Hydrochloride Injection orders are shipped under 2–8°C temperature-controlled conditions to preserve chemical stability and biological activity.
Packaging: Sterile vials are secured with thermal insulation and protective casing to prevent damage and light exposure.
Tracking: Each shipment includes tracking information for real-time monitoring.
Delivery Assurance: Guaranteed intact, sterile, and pharmacologically active product upon arrival.
Trade Assurance
Factory-Direct Supply: Sourced from GMP-compliant manufacturers with stringent quality control.
Lot-to-Lot Consistency: All batches validated with COA, TDS, MSDS to ensure reproducibility.
Global Compliance: Meets international standards for research-grade antineoplastic agents.
Customer Protection: Buyers are covered under trade agreements ensuring on-time delivery and product integrity.
Payment Support
Multiple Payment Options: T/T, PayPal, Western Union, and major international credit cards accepted.
Secure Transactions: Payments processed via verified and encrypted platforms.
Flexible Terms: Support for bulk orders, custom concentrations, and prepayment arrangements.
Disclaimer
Mitoxantrone Hydrochloride Injection (CAS 70476-82-3) is intended for research use only. It is not approved for human or veterinary clinical use. Researchers must adhere to institutional safety protocols, local regulations, and proper laboratory handling procedures. The manufacturer and supplier disclaim liability for misuse or clinical application outside controlled experimental conditions.
References
Gluck S. Mitoxantrone in the treatment of leukemia and lymphoma: mechanism and applications. Leuk Res. 1997;21:499–508. Link
Hartung HP, et al. Mitoxantrone in multiple sclerosis: mechanism of immunomodulation. Ann Neurol. 2002;51:71–78. Link
Weiss RB. Anthracenedione antineoplastic agents: pharmacology and toxicology. Cancer Treat Rev. 1992;19:77–96. Link
Curt GA, et al. Mechanistic studies of Mitoxantrone: DNA intercalation and topoisomerase II inhibition. Biochem Pharmacol. 1988;37:453–460. Link
Kantarjian HM, et al. Preclinical evaluation of Mitoxantrone in leukemia models. Blood. 1987;70:1105–1112. Link

Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 16 × 16 × 18 cm |
1. What is Mitoxantrone Hydrochloride Injection CAS 70476-82-3 used for in research?
It is primarily used in preclinical oncology research to study DNA intercalation, topoisomerase II inhibition, apoptosis induction, and cell cycle arrest in hematologic malignancies and solid tumor models.
2. How should Mitoxantrone Injection be stored?
Store at 2–8°C, protected from light. Avoid repeated freeze-thaw cycles to maintain chemical stability and pharmacological activity.
3. Can Mitoxantrone Hydrochloride be used in animal models?
Yes. It is suitable for murine, rat, and humanized models to study tumor progression, hematologic malignancies, and immune modulation. Dosing should be carefully monitored to prevent myelosuppression and cardiotoxicity.
4. What is the mechanism of action?
Mitoxantrone functions as a DNA intercalator and topoisomerase II inhibitor, inducing double-strand DNA breaks, cell cycle arrest in G2/M phase, and apoptosis. It also has immunomodulatory effects in autoimmune disease models.
5. What forms and concentrations are available?
Available as a sterile injectable solution with concentrations of 2 mg/mL, 4 mg/mL, 10 mg/mL, or customizable based on experimental requirements.
6. What is the shelf life of Mitoxantrone Injection?
Shelf life is 12–24 months under recommended 2–8°C storage conditions.
7. Is Mitoxantrone Injection safe for laboratory handling?
Yes, when used under Biosafety Level 2 (BSL-2) conditions, with protective equipment, proper disposal, and adherence to institutional safety protocols.
8. What are common side effects in preclinical studies?
Potential effects include myelosuppression, cardiotoxicity, mild gastrointestinal symptoms, and transient immune cell modulation. Dose-dependent effects should be monitored in long-term studies.
9. Can it be combined with other experimental drugs?
Yes. Commonly used with chemotherapy agents, immune modulators, DNA repair inhibitors, and novel topoisomerase II inhibitors to study synergistic cytotoxicity and drug resistance mechanisms.
10. Does it affect metabolism in experimental models?
High doses may mildly influence hepatic enzymes, metabolic pathways, and glucose metabolism, particularly in long-term animal studies. Researchers should control for systemic metabolic effects.
11. What documentation is provided with each batch?
Each batch includes COA (Certificate of Analysis), MSDS (Material Safety Data Sheet), TDS (Technical Data Sheet), and lot-specific data to ensure reproducibility and traceability.
12. How is Mitoxantrone Injection shipped?
Shipped under cold-chain conditions (2–8°C) with thermal insulation, ice packs, and tracking information to maintain sterility and pharmacological activity.
13. Is Mitoxantrone Hydrochloride approved for clinical use?
No. It is research-grade only and not for human or veterinary clinical applications. Use exclusively in laboratory or preclinical models.
14. Can custom concentrations or packaging be requested?
Yes. Vial sizes (1 mL, 5 mL, 10 mL) and bulk solutions are available, with OEM/private labeling options for research institutions.
15. What assays can be used to validate Mitoxantrone activity?
Activity can be confirmed via cytotoxicity assays, DNA damage assays (γ-H2AX, comet assay), topoisomerase II inhibition assays, and flow cytometry-based apoptosis measurements.

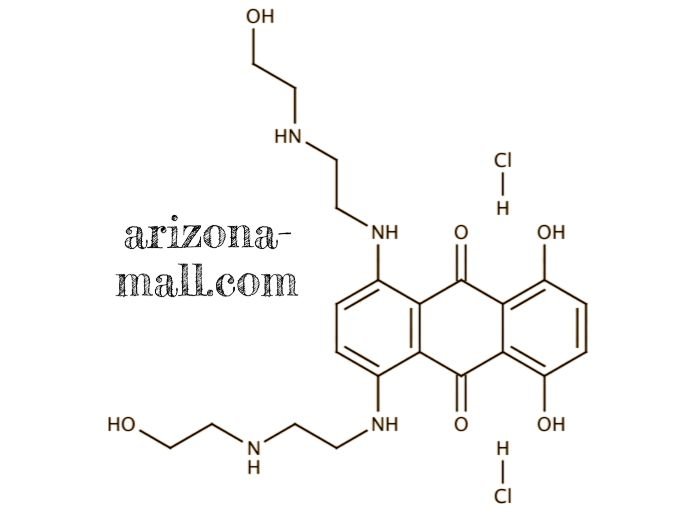











Reviews
There are no reviews yet.