No products in the cart.
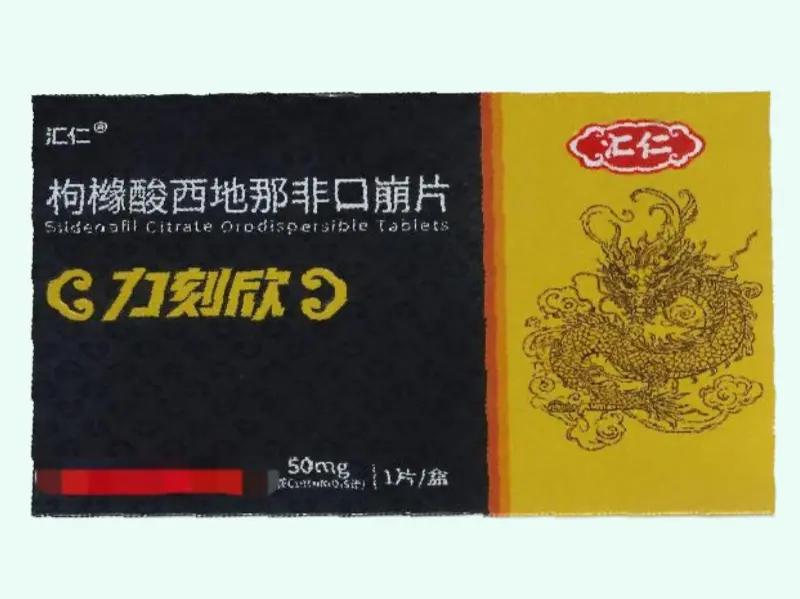
Sildenafil Citrate Orodispersible Tablet – Huiren / Likexin 50?mg
$1.00
Sildenafil Citrate is a selective phosphodiesterase?5 (PDE5) inhibitor used for erectile dysfunction and related vascular studies. This fast?acting orodispersible tablet (ODT) formulation delivers 50?mg per tablet, designed to disintegrate on the tongue without water. Ideal for laboratory and preclinical research—including pharmacokinetics, mechanism investigations, and andrology. Not approved for clinical, diagnostic, therapeutic, or veterinary use.
Description
Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Sildenafil Citrate Orodispersible Tablet (Huiren® / Likexin) |
| Generic Name | Sildenafil Citrate |
| CAS Number | 171599?83?0 patents.google.com+4gz.zbzxyy.com+4nmpa.gov.cn+4 |
| Molecular Formula | C??H??N?O?S · C?H?O? |
| Molecular Weight | ?666.7?g/mol |
| Dosage Form | Oro?dispersible tablet (ODT) |
| Strength | 50?mg per tablet |
| Pack Size | 1 tablet per box |
| Approval Number | China NMPA H20244673 |
| Product Code | 86910323000369 |
| Manufacturer | Jiangxi Huiren Pharmaceutical Co., Ltd. |
| Barcode | 6937999607160 |
| Storage | Store at 15–25?°C; protect from light and moisture |
| Intended Use | Laboratory research only |
Mechanism of Action
Sildenafil selectively inhibits PDE5, preventing breakdown of cGMP in smooth muscle cells, leading to enhanced nitric oxide (NO)-mediated vasodilation, especially in penile and pulmonary vasculature.
Research Applications & Pharmacology
Erectile Dysfunction (ED) Models: Widely used to assess cGMP-mediated signaling and vascular responses .
Pulmonary Arterial Hypertension: Employed to investigate PDE5’s role in pulmonary vascular dilation.
Pharmacokinetics of ODTs: Orodispersible formulations dissolve rapidly in seconds—promoting fast absorption without water .
Clinical Parameters: Typical onset ~20 minutes, duration ~2 hours; metabolic clearance by CYP3A4/5; excreted primarily in feces and urine .
Safety Profile & Handling
Common Adverse Effects: Headache, flushing, nasal congestion, dyspepsia, and mild dizziness .
Drug Interactions: Avoid concurrent administration with nitrates or NO donors due to risk of severe hypotension .
Contraindications: Not suitable for models involving cardiovascular compromise or concurrent PDE5-inhibiting agents.
Handling Instructions: Store under recommended conditions, use protective PPE, discard as per institutional safety protocols. This product is strictly for research and not for administration.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |












Reviews
There are no reviews yet.