No products in the cart.
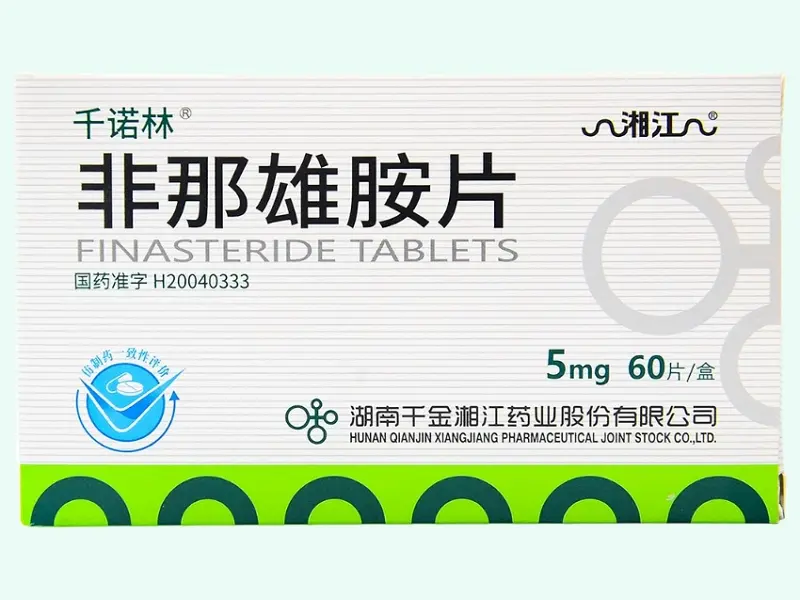
Finasteride Tablets (Qianolin® / Xiangjiang) 5?mg ×?60 Tablets
$1.00
Finasteride is a selective 5?-reductase inhibitor that prevents testosterone from converting to dihydrotestosterone (DHT), widely used in biomedical research on androgenic alopecia and benign prostatic hyperplasia (BPH). This product offers 5?mg tablets in a 60-count bottle from Hunan Qianjin Xiangjiang Pharmaceutical. Strictly for laboratory or preclinical research use only—not for therapeutic, clinical, diagnostic, or veterinary application.?Please consult staff for other specifications and uses?
Description
Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Finasteride Tablets (Qianolin® / Xiangjiang) |
| Generic Name | Finasteride |
| CAS Number | 98319?26?7 (zh.wikipedia.org) |
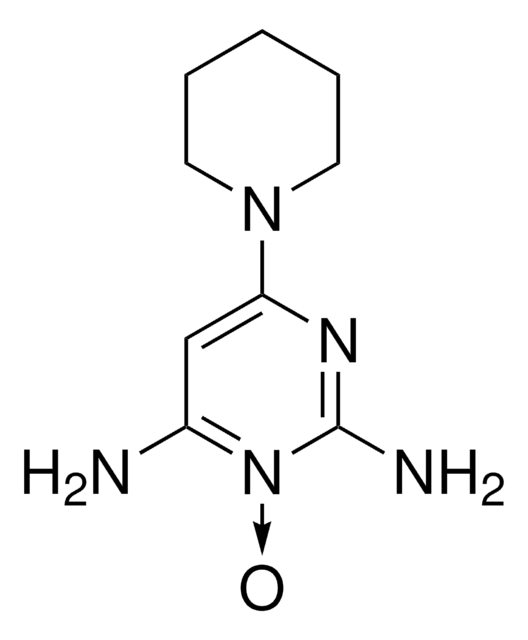
| Molecular Formula | C??H??N?O? |
| Molecular Weight | ~372.5?g/mol (free base) |
| Dosage Form | Film-coated oral tablet |
| Strength | 5?mg per tablet |
| Pack Size | 60 tablets per bottle |
| Approval No. | China NMPA H20040333 |
| Product Code | 86904960000424 |
| Manufacturer | Hunan Qianjin Xiangjiang Pharmaceutical Co., Ltd. |
| Barcode | 6934805201703 |
| Storage Conditions | Store at 15–25?°C; keep dry and away from light |
| Intended Use | Laboratory/preclinical research only |
Mechanism of Action
Finasteride is a competitive inhibitor of Type II (and III) 5?-reductase, blocking the conversion of testosterone to DHT and reducing serum and tissue levels of DHT by approximately 70%–90% . This enzyme blockade is central to androgenic alopecia and BPH models.
Research Applications & Pharmacology
Androgenic Alopecia Studies: Suppresses follicular DHT, slowing hair loss and promoting regrowth .
Benign Prostatic Hyperplasia (BPH) Models: Reduces prostate volume, urinary retention, and delays surgical intervention.
Pharmacokinetics: ~90% protein bound, bioavailability ~65%, half-life ~5–6?hours in adults (longer in elderly); metabolized by liver (CYP3A4), excreted in feces (57%) & urine (40%) .
Comparative 5-ARI Research: Finasteride inhibits Type II, leading to ~70% DHT reduction, vs. dutasteride (~95%) .
Safety Profile & Handling
Common Adverse Effects (in vivo): Rare sexual dysfunction, decreased libido, ejaculation disorders .
Serious Risks: Potential depression, self-harm (in elderly), gynecomastia (~1.3%) .
Contraindications: Not for use by females, especially pregnant, due to teratogenic DHT suppression.
Handling Advice: Store under recommended temperature; use PPE when opening; discard per lab biosafety guidelines.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |












Reviews
There are no reviews yet.