No products in the cart.
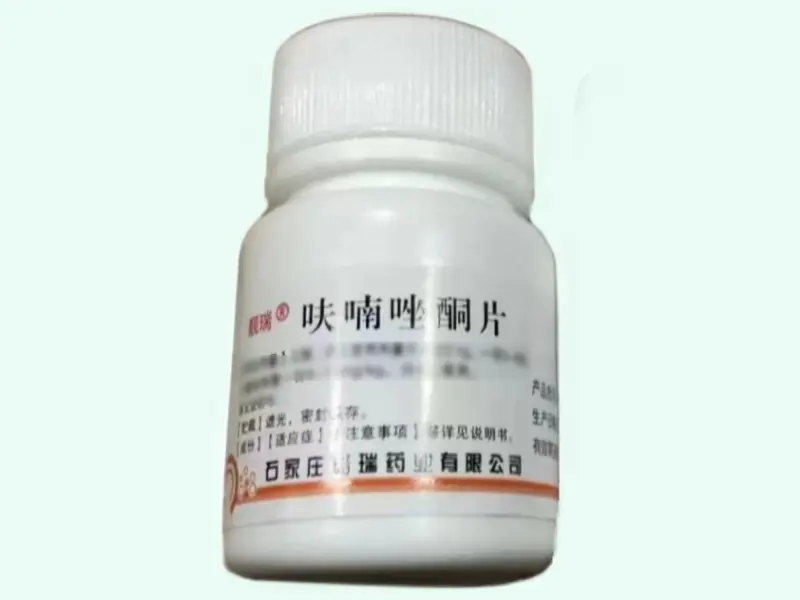
Furazolidone Tablets 0.1 g × 100 Tablets (Liangrui)
$2.00
Furazolidone Tablets (brand name: Liangrui) contain 0.1 g of furazolidone per tablet and are intended for laboratory research applications, particularly in microbiological and pharmacological studies. Packaged as 100 tablets per bottle, this product is manufactured by Shijiazhuang Gree Pharmaceutical Co., Ltd. Registered under Chinese NMPA approval H13020115.?? Strictly for laboratory research use only.?Please consult staff for other specifications and uses?
Description
Furazolidone Tablets Product Specifications
| Specification | Description | Synonyms / Alternative Names |
|---|---|---|
| Product Name | Furazolidone Tablets (Liangrui) | Furazolidone, Furazol, 3-(5-nitrofurfurylideneamino)-2-oxazolidinone |
| Generic Name | Furazolidone | Furazolidone |
| Formulation | Oral tablet | Tablet |
| Strength | 0.1 g (100 mg) per tablet | 100 mg |
| Pack Size | 100 tablets per bottle | Bottle |
| Approval Number | H13020115 (NMPA, China) | NMPA Approval |
| Product Code | 86902762000215 | |
| Manufacturer | Shijiazhuang Gree Pharmaceutical Co., Ltd. | |
| Barcode | 6933982500999 | |
| Storage Conditions | Store below 30?°C in a dry place | |
| Intended Use | For laboratory research only |
Furazolidone Tablets Mechanism of Action
Furazolidone is a nitrofuran antibiotic that exerts antimicrobial effects by interfering with bacterial enzyme systems and DNA synthesis. It is widely used in research to study antibacterial resistance, microbial growth inhibition, and pharmacodynamics.
Research Applications & Pharmacology
In vitro and in vivo antimicrobial research
Studies on bacterial resistance mechanisms
Experimental pharmacology on nitrofuran derivatives
Microbiological testing and assay development
Safety Profile & Handling
Potential irritant; use lab PPE (gloves, mask, lab coat)
Avoid inhalation, skin and eye contact
Store in a dry, well-ventilated place away from sunlight
Follow hazardous waste disposal protocols
Core Keywords
Furazolidone, Liangrui, nitrofuran antibiotic, antimicrobial research, laboratory chemical, bacterial resistance studies, experimental tablets
Research-Use Disclaimer
This product is intended solely for laboratory research use. It is not approved for clinical, therapeutic, diagnostic, or veterinary applications. Improper handling or unauthorized use may result in health hazards.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 25 × 26 × 25 cm |












Reviews
There are no reviews yet.