No products in the cart.
Drotaverine Hydrochloride Injection 40?mg (Duoli – Shenghuaxi) – Wholesale price comparison
$2.00
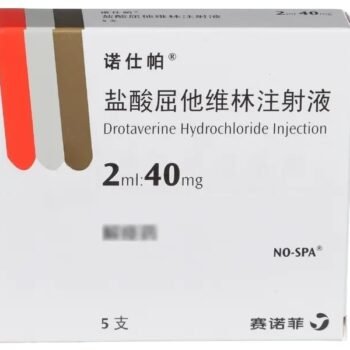
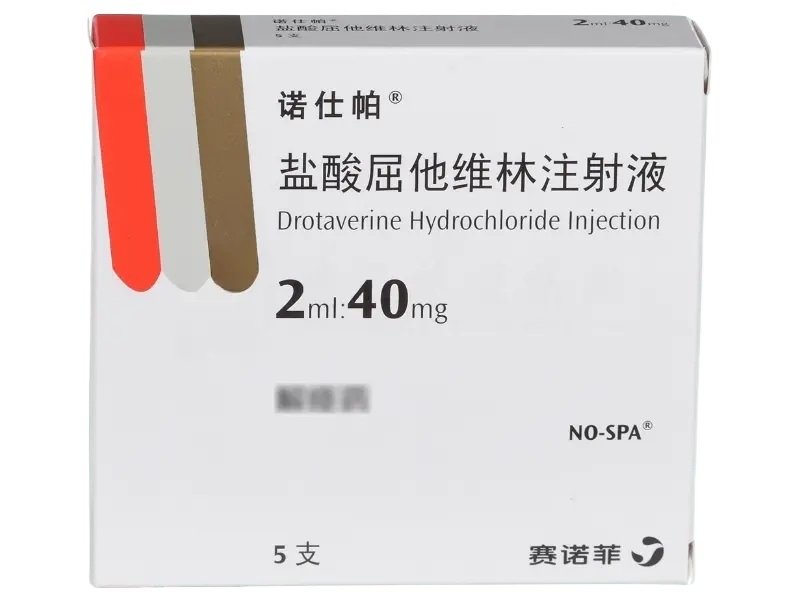
Duoli (Shenghuaxi) Drotaverine Hydrochloride Injection delivers 40?mg per 2?ml ampoule, 5 ampoules per box. Produced by Chongqing Shenghuaxi Pharmaceutical Co., Ltd. and NMPA-approved under HJ20183353. Ideal for laboratory studies on smooth muscle relaxation in gastrointestinal, biliary, genitourinary, and vascular systems.
?? For laboratory and research use only.?Please consult staff for other specifications and uses?
Description
Drotaverine is a benzylisoquinoline antispasmodic that works by selectively inhibiting phosphodiesterase-4 (PDE?4), thereby increasing cyclic AMP levels and promoting smooth muscle relaxation. It is widely used in research settings to study spasm relief in visceral organs, uterine tissue, and blood vessels due to its rapid onset and vasodilatory properties.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Drotaverine Hydrochloride Injection (Duoli / Shenghuaxi) |
| Active Ingredient | Drotaverine Hydrochloride |
| CAS Number | 14009?24?6 |
| Molecular Formula | C??H??NO? |
| Molecular Weight | ~397.5?g/mol |
| Formulation | Aqueous solution for injection, 2?ml ampoules |
| Strength per Ampoule | 40?mg |
| Pack Size | 5 ampoules per box |
| Dosage Form | Injectable solution |
| Approval Number | HJ20183353 |
| Drug Code | 86909568000197 |
| Manufacturer | Chongqing Shenghuaxi Pharmaceutical Co., Ltd. |
| Barcode | 6933982500616 |
| Storage Conditions | Store at 20–25?°C, protected from light and moisture |
| Intended Use | Laboratory research on smooth muscle spasm and vascular relaxation |
Mechanism of Action
Drotaverine inhibits PDE?4, elevating intracellular cAMP, activating PKA, and leading to myosin light-chain kinase inhibition, which results in smooth muscle relaxation. It also contributes to vasodilation and blood flow enhancement.
Research Applications
Gastrointestinal and biliary colic models
Ureteric and urinary smooth muscle spasm studies
Uterine relaxation and dysmenorrhea models
Vascular smooth muscle and hemodynamic research
Comparative studies of smooth muscle relaxants
Safety & Handling
Dosage in animal studies: typically 40–80?mg IV or IM, once or twice daily.
Side effects: possible headache, dizziness, nausea; rare arrhythmia or hypotension.
Contraindications: hypersensitivity to drotaverine; caution in cardiovascular compromise.
Handling: Use lab PPE. Avoid needle-stick or aerosol exposure.
Storage: Keep sealed at 20–25?°C, protected from light and moisture.
Core Keywords
drotaverine injection, drotaverine hydrochloride, No?Spa analog, CAS 14009?24?6, HJ20183353, PDE?4 inhibitor injection, smooth muscle relaxant, biliary colic research, wholesale drotaverine ampoules
Research Use Disclaimer
This product is strictly for laboratory and research use. It is not approved for clinical, therapeutic, diagnostic, or veterinary use. Misuse may pose legal or health risks. Use only under approved institutional protocols.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |













tssta –
Bedankt, de service is erg goed