No products in the cart.
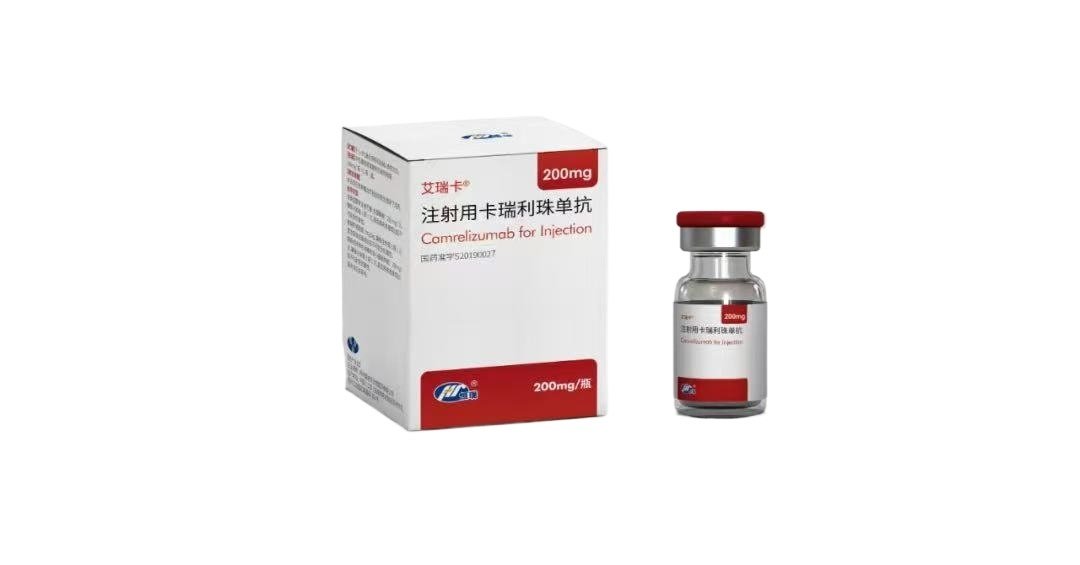
Camrelizumab Injection 200?mg – Low price wholesale comparison
$1.00
Camrelizumab Injection (Zhengguo) provides 1100?mg of humanized IgG4 anti?PD?1 monoclonal antibody in an 11?mL vial. Developed by Alexion (Ireland) and approved under China NMPA license SJ20250016. Strictly for laboratory research use?– wholesale and retail orders available.?Please consult staff for other specifications and uses?
Description
Camrelizumab (SHR?1210) is a humanized, high-affinity IgG4 ? monoclonal antibody targeting programmed cell death protein?1 (PD?1). By disrupting PD?1/PD?L1 interactions, it reinvigorates T and B lymphocytes and NK cells, enabling immunologic cancer cell recognition?. Widely studied in over 5,000 patients across multiple Phase?II/III trials, camrelizumab has shown promising antitumor activity, notably in hepatocellular carcinoma and Hodgkin lymphoma?.
Developed by Alexion Pharma International at the Athlone facility in Ireland, this vial is authorized for research use. China NMPA registration is SJ20250016, drug code 86982467000031. ? For lab research only—not for therapeutic or diagnostic use.
Camrelizumab Injection Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Camrelizumab Injection (Zhengguo) |
| Generic Name | Camrelizumab (SHR?1210) |
| CAS Number | 1798286?48?2 |
| Molecular Type | Humanized IgG4 ? monoclonal antibody |
| Mechanism of Action | PD?1 immune checkpoint inhibition |
| Dosage Form | Sterile injectable solution (vial) |
| Strength | 200?mg in 11?mL vial |
| Packaging | 1 vial per box |
| Approval Number | SJ20250016 (China NMPA) |
| Product Code | 86982467000031 |
| Manufacturer | Alexion Pharma International Operations (Ireland) |
| Barcode | Pending – contact for registration |
| Storage Conditions | Store at 2–8?°C; protect from light |
| Intended Use | Laboratory research only |
Camrelizumab Injection Mechanism & Research Applications
Camrelizumab binds PD?1 receptors on T, B, and NK cells, blocking their inhibition by PD?L1, and restoring anti?tumor immunity?. It has been used extensively in:
Immuno-oncology studies (hepatocellular carcinoma, melanoma, Hodgkin lymphoma)
Combination studies with VEGFR inhibitors and chemotherapy?
Investigations into immune-related adverse events like cutaneous capillary proliferation?
Biomarker research (PD?L1 expression, T-cell activation assays)
Camrelizumab Injection Side Effects (Research Observations)
Based on clinical trial data, common immune-related events include:
Reactive cutaneous capillary endothelial proliferation (RCCEP) – typically mild and reversible?
Infusion-associated effects: fever, fatigue, rash, pruritus
Rare immune-related organ toxicities: hepatitis, colitis, pneumonitis
Include immune toxicity monitoring when used in animal models.
Safety & Handling
Use Restriction: For laboratory research only – not for human or veterinary use
Handling: Use aseptic technique and lab-grade PPE (gloves, gown, eye protection)
Storage: Between 2–8?°C; protect from light
Disposal: Dispose as per institutional biohazard and pharmaceutical regulations
Core Keywords
Camrelizumab injection, Zhengguo camrelizumab, anti?PD?1 monoclonal antibody, immuno-oncology research, lab-grade PD?1 inhibitor, 1100?mg camrelizumab vial, NMPA SJ20250016 camrelizumab, Alexion camrelizumab, wholesale camrelizumab injection
Research Use Disclaimer
This product is intended exclusively for laboratory research use and is not approved for diagnostic, therapeutic, clinical, or veterinary applications. Misuse may pose safety or regulatory concerns. Always follow institutional biosafety and handling guidelines.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |













abd.el –
Thank you, I have received the package