No products in the cart.
Gamifant (emapalumab?lzsg) 10?mg – For Research Use Only
$2.00
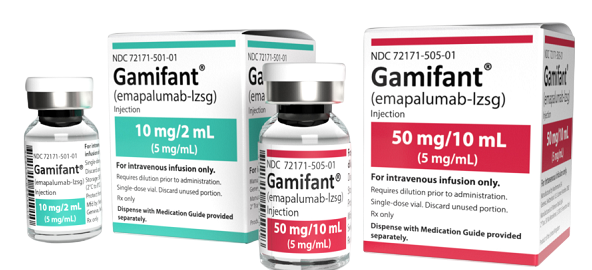
Gamifant (emapalumab?lzsg) is a human IgG1 monoclonal antibody that neutralizes interferon?gamma (IFN?). Supplied as 10?mg in 2?ml sterile injection vial. Produced by Patheon Italia under Chinese approval SJ20220008. Used in research on cytokine storm, macrophage activation, hemophagocytic lymphohistiocytosis (HLH) modeling. For laboratory research use only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
Emapalumab?lzsg (branded as Gamifant) is the first IFN??neutralizing antibody approved for treating primary hemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS). In research, it serves as a potent tool to investigate hyperinflammatory disorders, IFN?-driven cytokine storm, macrophage activation, and immune dysregulation. It enables study designs involving immune suppression, CAR?T?related neurotoxicity models, and cytokine modulation experiments. For laboratory research use only.
Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Gamifant (emapalumab?lzsg) Injection |
| Synonyms | Emapalumab; IFN??neutralizing antibody |
| Strength | 10?mg per vial (2?ml, 5?mg/ml) |
| Dosage Form | Intravenous injection vial |
| Packaging | 1 vial per box |
| Manufacturer | Patheon Italia S.p.A. (Italy) |
| Approval Number | SJ20220008 |
| Drug Standard Code | 86984083000013 |
| Barcode | Not yet assigned |
| CAS Number | Not assigned (monoclonal antibody complex) |
| Molecular Type | Human IgG1 monoclonal antibody (~148?kDa) |
Mechanism of Action & Research Applications
Emapalumab?lzsg binds to and neutralizes interferon?gamma, inhibiting downstream signaling pathways that drive hyperinflammation. It is particularly suited for research into cytokine-driven immune activation, HLH and MAS models, CAR?T cell therapy–related toxicity, and the pharmacodynamics of immune suppression via IFN? blockade.
Side Effects (For Reference Only in Research Models)
Analogous clinical observations include infection risk (especially viral), infusion-related reactions, hypertension, fever, and hematologic changes. These inform model safety and dosing protocols in experimental designs.
Disclaimer
Gamifant (emapalumab?lzsg) is strictly intended for laboratory research use only. Not for therapeutic, diagnostic, or prophylactic use in humans or animals.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |











Reviews
There are no reviews yet.