No products in the cart.
Sale
Teverelix (CAS 151272-78-5) | GMP-Grade GnRH Antagonist Peptide for Urology & Endometriosis Research
Original price was: $22.00.$18.00Current price is: $18.00.
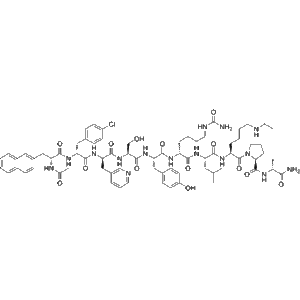
Teverelix, a synthetic decapeptide GnRH antagonist (CAS 151272-78-5), competitively and reversibly binds GnRH receptors to suppress LH and FSH. Manufactured under GMP, it’s ideal for research into prostatic hyperplasia, endometriosis, and prostate cancer models. For laboratory research only.
Description
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Teverelix (EP 24332) |
| CAS Number | 151272-78-5 |
| Synonyms | Antarelix, EP 24332 |
| Molecular Formula | C??H???ClN??O?? |
| Molecular Weight | ~1459.13 Da |
| Purity | ? 98% (HPLC) |
| Mechanism | GnRH receptor competitive & reversible antagonist |
| Target Hormones | Suppresses LH, FSH, testosterone |
| Applications | Prostatic hyperplasia, endometriosis, prostate cancer research |
| Appearance | Lyophilized peptide powder |
| Storage | –20 °C (powder); in DMSO: –80 °C (6 mo) / –20 °C (2 weeks) |
| GMP Compliance | Manufactured under GMP standards |
Product Description
Teverelix is a synthetic decapeptide GnRH antagonist designed for reversible, competitive inhibition of gonadotropin-releasing hormone receptors. By blocking GnRH signaling at the pituitary gland, it effectively suppresses the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which consecutively inhibits sex hormone production such as testosterone and estradiol .
Investigated in Phase II clinical trials (e.g., trials EP-24332T series), Teverelix demonstrated rapid suppression of LH, FSH, and testosterone, achieving “castrate” testosterone levels within two days post-dosing. Dose regimens included subcutaneous (SC) administration over consecutive days, leading to sustained hormone suppression—making it compelling for translational research models targeting hormone-driven conditions.
With high GMP manufacturing standards and ?98% purity, Teverelix offers researchers stability and reproducibility. Whether studying benign prostatic hyperplasia (BPH), prostate cancer, or endometriosis mechanisms, Teverelix provides a precise tool for hormone axis modulation in both in vitro and in vivo settings.
Mechanism of Action & Research Applications
Teverelix functions as a competitive, reversible GnRH receptor antagonist. It blocks GnRH binding to pituitary GnRH receptors, preventing the downstream release of LH and FSH, and subsequently lowering sex steroid levels.
Key Research Applications:
Prostatic Hyperplasia & LUTS: In a phase II trial (EP-24332T-A012), Teverelix was administered SC on Day 1 and Day 3, leading to significant symptom relief (IPSS score reductions), improved peak urine flow, and reduced prostate volume—highlighting its potential in BPH/LUTS models.
Prostate Cancer Research: Rapid testosterone suppression is critical in preclinical prostate cancer models. Teverelix’s quick and sustained castrate effect provides a useful mechanism to explore androgen deprivation strategies and tumor response.
Endometriosis & Reproductive Research: Since LH/FSH signaling contributes to endometrial proliferation, Teverelix can be used to study hormone regulation in endometriosis and reproductive studies.
Pharmacokinetics/Pharmacodynamics Modeling: Teverelix exhibits a distinctive PK profile with initial burst and prolonged release phases, ideal for pharmacometric modeling and dose optimization studies antev.co.uk.

Side Effects (For Reference in Research Models)
In clinical Phase II trials, Teverelix was generally well-tolerated. Key observations included:
Injection-site reactions: Mild and self-resolving induration, particularly following SC administration due to depot formation.
Systemic Effects: As with typical GnRH antagonism, effects such as hot flushes or hormone withdrawal symptoms can be anticipated in preclinical models.
Model-specific variability: Differences between SC and IM delivery affect onset and duration of hormone suppression, important for experimental design.
Disclaimer
is supplied exclusively for laboratory research use. Not intended for clinical, veterinary, or human use. Users should follow appropriate safety protocols and institutional guidelines.
Keywords
Cas 151272-78-5; GnRH antagonist peptide; LH FSH suppression; GnRH receptor blocker; BPH research peptide; Prostate cancer research; Endometriosis research; GMP teverelix supplier; Laboratory hormone research peptide.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 63 × 26 × 63 cm |












Reviews
There are no reviews yet.