No products in the cart.
Sale
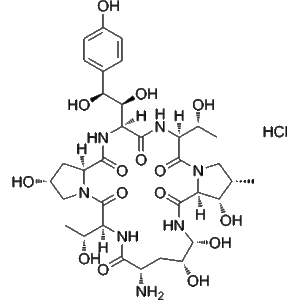
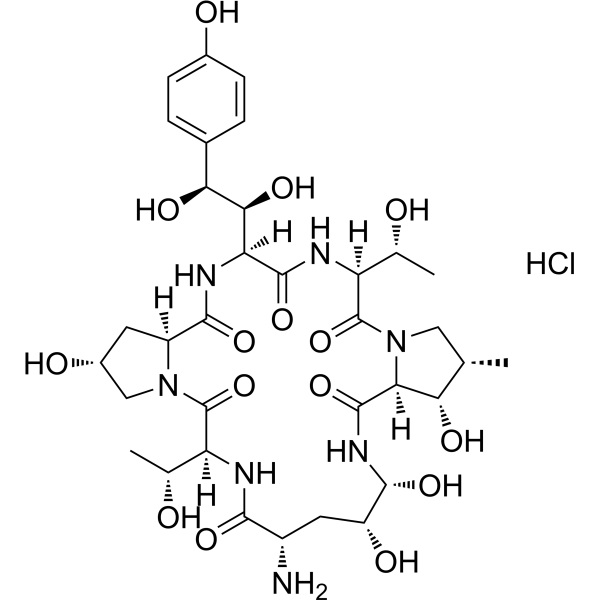
A-30912A nucleus hydrochloride | CAS 1029890-89-8 | powder raw material manufacturers supply wholesale
Original price was: $33.00.$26.00Current price is: $26.00.
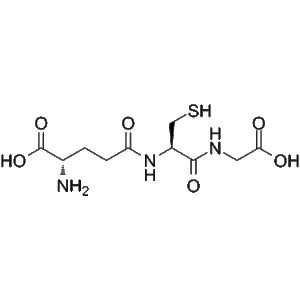
A-30912A nucleus hydrochloride (CAS 1029890-89-8) is an enzymatically deacylated derivative of Echinocandin B, produced via ECB deacylase catalysis. It serves as a valuable intermediate for antifungal biosynthesis and echinocandin mechanism studies.
Description
Product Description
A-30912A nucleus hydrochloride is a specialized biochemical compound obtained through the enzymatic deacylation of Echinocandin B (ECB) by the enzyme Echinocandin B deacylase. This enzymatic conversion generates the core structure, or “nucleus,” of A-30912A, a member of the echinocandin family of antifungal lipopeptides. The compound represents an essential intermediate in the biosynthesis and modification of echinocandin-type antifungal agents, which target fungal cell wall synthesis by inhibiting β-(1,3)-D-glucan synthase.
Background and Origin
Echinocandins are cyclic lipopeptides originally derived from fungal fermentation processes. They exhibit potent antifungal activity by disrupting cell wall formation in susceptible fungi, particularly Candida and Aspergillus species. The natural product Echinocandin B serves as a precursor in the production of several clinically relevant antifungal agents such as caspofungin and micafungin. During biosynthesis, selective enzymatic deacylation yields the A-30912A nucleus, which retains the cyclic peptide backbone but lacks the lipid side chain responsible for pharmacokinetic properties.
A-30912A nucleus hydrochloride is therefore a structurally and functionally pivotal intermediate. Researchers utilize it to study how enzymatic transformations, particularly deacylation and reacylation, influence antifungal activity, molecular stability, and specificity for β-glucan synthase. By studying this compound, scientists gain insights into the synthetic pathways and mechanism-based optimization of echinocandin derivatives.
Role in Research
The A-30912A nucleus provides an ideal scaffold for the design and synthesis of new antifungal agents. Because it contains the essential cyclic hexapeptide core conserved across echinocandin compounds, it can undergo chemical or enzymatic modification to produce analogs with altered lipophilic moieties or improved stability. Its use allows for systematic exploration of structure–activity relationships (SAR) in the echinocandin class and enables fine-tuning of antifungal potency, selectivity, and metabolic stability.
In addition, this compound serves as a biochemical tool in studies of enzyme catalysis. The enzyme Echinocandin B deacylase catalyzes the cleavage of the fatty acid side chain, producing A-30912A nucleus hydrochloride. By investigating this enzymatic step, researchers can elucidate how deacylases control substrate specificity, stereochemistry, and reaction kinetics. Such studies have broad implications for microbial biotransformation, industrial enzymology, and biosynthetic pathway engineering.
Relevance to Antifungal Mechanism Studies
Echinocandins act by non-competitively inhibiting β-(1,3)-D-glucan synthase, an enzyme essential for the synthesis of glucan polymers in fungal cell walls. A-30912A nucleus hydrochloride retains the peptide framework necessary for binding to the enzyme’s active site. Thus, it is used as a biochemical probe to investigate how modifications in side chain length, polarity, or charge affect binding affinity and inhibitory potency.
Beyond structural biology, this compound is also useful in bioengineering and synthetic biology contexts. Through microbial or enzymatic modification of A-30912A nucleus, novel derivatives can be generated that expand the structural diversity of the echinocandin class. These analogs are then tested for improved antifungal efficacy or reduced resistance development.
Practical Laboratory Applications
In the laboratory, A-30912A nucleus hydrochloride is often employed as a reference standard in HPLC or LC-MS analyses of echinocandin biosynthetic products. It provides a reliable molecular fingerprint for confirming the success of enzymatic deacylation reactions. Moreover, because it exists as a hydrochloride salt, the compound exhibits improved solubility in aqueous and polar organic solvents, facilitating its handling in biochemical assays.
Researchers engaged in antifungal biosynthesis, metabolic pathway elucidation, or drug development use this compound as a benchmark substrate for assessing enzymatic selectivity and catalytic efficiency. The reproducible preparation and high purity (≥99%) ensure consistency across experiments, which is vital for both analytical and preparative-scale studies.
In summary, A-30912A nucleus hydrochloride is a cornerstone compound for understanding echinocandin biosynthesis and antifungal mechanism research. Its role as both a natural product intermediate and a biochemical probe makes it indispensable in academic and industrial laboratories investigating fungal cell wall inhibitors, enzymatic catalysis, and antibiotic optimization strategies.
Product Specifications
| Parameter | Specification |
|---|---|
| Product Name | A-30912A nucleus hydrochloride |
| CAS Number | 1029890-89-8 |
| Synonyms | Echinocandin B deacylated nucleus, A-30912A hydrochloride |
| Molecular Formula | C₅₀H₇₁ClN₉O₁₈ |
| Molecular Weight | 1116.6 g/mol |
| Purity | ≥99% |
| Appearance | White to off-white solid |
| Solubility | Soluble in DMSO, methanol, and slightly soluble in water |
| Storage Conditions | -20°C, protected from light and moisture |
| Applications | Antifungal biosynthesis studies, echinocandin derivative mechanism research, enzyme catalysis modeling, β-glucan synthase inhibition research |
Mechanism of Action
A-30912A nucleus hydrochloride functions as both a biochemical intermediate and a mechanistic model in antifungal research. Its biological and structural significance stems from its role as the deacylated form of Echinocandin B, produced via Echinocandin B deacylase-catalyzed hydrolysis.
Enzymatic Deacylation Process
In the biosynthesis of echinocandins, the lipid acyl side chain attached to the cyclic peptide core plays a major role in determining antifungal potency, pharmacokinetics, and cell membrane affinity. The deacylation reaction catalyzed by ECB deacylase selectively removes this lipid moiety, generating the A-30912A nucleus. This transformation is not merely a structural alteration but a key biosynthetic control point that allows for subsequent chemical or enzymatic reacylation to yield diverse echinocandin analogs.
Through mechanistic analysis of this reaction, researchers can study enzyme–substrate interactions, stereochemical preferences, and transition state stabilization. Such knowledge contributes to the rational engineering of enzymes capable of producing novel echinocandin derivatives with improved biological profiles.
Interaction with β-(1,3)-D-glucan Synthase
Although A-30912A nucleus hydrochloride itself is not typically used as a therapeutic antifungal, it retains the cyclic hexapeptide structure necessary for weak interaction with β-(1,3)-D-glucan synthase. This interaction allows researchers to investigate how conformational changes in the peptide ring influence binding kinetics, enzyme inhibition, and structural rigidity. These studies help refine the understanding of the echinocandin mechanism, where selective binding to glucan synthase inhibits fungal cell wall synthesis and leads to osmotic instability and fungal cell death.
Application in Structure–Activity Relationship (SAR) Studies
By comparing A-30912A nucleus to fully acylated echinocandin derivatives, scientists can elucidate the role of lipid moieties in membrane association, hydrophobic binding, and pharmacological activity. The nucleus serves as a molecular template to which varied acyl groups can be added, allowing systematic SAR mapping. Such research drives the discovery of new echinocandin analogs with optimized stability, spectrum, and potency.
Biochemical and Synthetic Biology Applications
In biotechnological research, the deacylation reaction producing A-30912A nucleus hydrochloride is exploited for biosynthetic pathway engineering. By cloning and expressing the echinocandin deacylase enzyme in heterologous hosts, researchers can reproduce this reaction in microbial systems, enabling scalable production of intermediates for downstream modification. This enzymatic approach aligns with green chemistry principles by minimizing chemical reagents and optimizing reaction specificity.
Functional Insights
The study of A-30912A nucleus hydrochloride contributes to understanding:
the modular enzymology of lipopeptide biosynthesis,
the structural determinants of β-glucan synthase inhibition,
and the biochemical adaptability of echinocandin scaffolds.
Collectively, these insights provide a foundation for rational antifungal design, where enzymatic and chemical modifications of the nucleus can yield next-generation antifungal compounds with reduced resistance potential and improved pharmacodynamic properties.
Side Effects
In laboratory and biochemical research contexts, A-30912A nucleus hydrochloride exhibits low toxicity and minimal hazard potential. Because it is a deacylated peptide intermediate rather than a fully active antifungal agent, it demonstrates limited interaction with mammalian systems. However, as a biologically active peptide analog, it should be handled with care under appropriate laboratory safety protocols.
Potential side effects observed in cellular assays may include mild alterations in membrane integrity at high concentrations due to peptide amphiphilicity. In enzyme assays, excessive concentrations may cause nonspecific inhibition of unrelated hydrolases or esterases. These effects are typically concentration-dependent and reversible upon dilution.
The compound should not be used for therapeutic purposes or administered to humans or animals. It is intended solely for in vitro biochemical, enzymological, and antifungal mechanism studies. Standard laboratory safety precautions — including use of gloves, lab coats, and fume hoods — are recommended.
Keywords
A-30912A nucleus hydrochloride, Echinocandin B deacylated nucleus, antifungal biosynthesis intermediate, echinocandin mechanism research, β-glucan synthase inhibitor, enzyme-catalyzed deacylation, lipopeptide biosynthesis, antifungal structure–activity relationship, echinocandin derivative, ECB deacylase substrate
Shipping Guarantee
All shipments are handled using validated cold-chain logistics to preserve peptide integrity. Each package is sealed in moisture-proof containers with secondary protective wrapping and continuous temperature monitoring. Products are shipped via express international couriers with full tracking and insurance coverage.
Trade Assurance
We ensure product authenticity, verified ≥99% purity, and compliance with analytical standards (HPLC, MS, and NMR). Each batch is supplied with a Certificate of Analysis (CoA). Our trade assurance policy guarantees replacement or refund for any deviation from listed specifications.
Payment Support
We provide flexible and secure global payment options to support international research transactions. Accepted payment methods include PayPal, major credit cards (Visa, MasterCard, American Express), telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are protected by industry-standard encryption and verified payment gateways to ensure confidentiality and fund security.
Disclaimer
All products listed are intended for laboratory research use only and not for human or veterinary use. They are not drugs, medical devices, or diagnostics and should not be administered to humans or animals. Researchers must handle all materials in accordance with institutional biosafety and chemical safety guidelines. The information provided is for scientific reference only and does not imply therapeutic efficacy, safety, or regulatory approval.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 65 × 45 × 65 cm |
What is A-30912A nucleus hydrochloride?
It is a deacylated derivative of Echinocandin B, produced by ECB deacylase, used in antifungal biosynthesis and mechanism research.
What is its CAS number?
CAS No. 1029890-89-8.
What is the purity level?
The product purity is ≥99%.
What are the main research applications?
Antifungal biosynthesis, echinocandin derivative mechanism studies, and enzyme catalysis research.
What enzyme produces it?
It is generated via the enzymatic action of Echinocandin B deacylase.
Is A-30912A nucleus hydrochloride an active antifungal?
No, it is a biosynthetic intermediate used for research, not a therapeutic compound.
How should it be stored?
At -20°C, protected from moisture and light.
Is it soluble in water?
Slightly soluble in water, readily soluble in DMSO and methanol.
Is it safe for human use?
No, it is strictly for laboratory research use only.
What type of studies benefit from using this compound?
Studies focusing on antifungal biosynthesis, enzymatic deacylation, and echinocandin structure–activity relationships.











Reviews
There are no reviews yet.