No products in the cart.
Sale
9-Valent HPV Vaccine | Recombinant Prophylactic Vaccine for HPV Research
Original price was: $3.00.$2.00Current price is: $2.00.
The 9-Valent HPV Vaccine is a recombinant, non-infectious, virus-like particle (VLP) vaccine developed for laboratory and preclinical research focused on human papillomavirus (HPV) prevention and immunology. It provides broad antigenic coverage against nine HPV genotypes (types 6, 11, 16, 18, 31, 33, 45, 52, and 58), enabling research on cross-type immune responses, antibody generation, and neutralizing mechanisms.
Description
Contents
hide
Product Description
The 9-Valent Human Papillomavirus (HPV) Vaccine represents a major advancement in the field of immunological and virological research. It is designed to provide broad-spectrum coverage against nine key HPV genotypes—types 6, 11, 16, 18, 31, 33, 45, 52, and 58—that are associated with both low-risk and high-risk HPV infections.
In laboratory research settings, this vaccine serves as a model system to explore the structural immunogenicity of L1 virus-like particles (VLPs), humoral immune response induction, and cross-neutralization across related HPV strains.
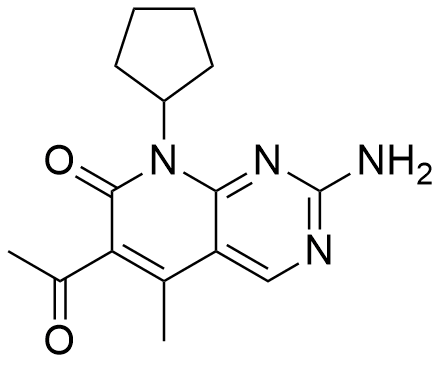
The vaccine formulation consists of highly purified recombinant L1 capsid proteins self-assembled into non-infectious VLPs, produced using a yeast-based expression system. These VLPs closely mimic the native structure of HPV virions without containing viral DNA, ensuring biosafety for laboratory manipulation.
Each HPV type in the 9-valent formulation contributes to the understanding of multivalent immune synergy and antigenic dominance patterns. Researchers utilize this preparation to examine B-cell activation, antibody avidity, and immune memory following multivalent antigen exposure.
The 9-Valent HPV Vaccine provides a vital tool for:
Studying vaccine-induced immunogenicity across multiple HPV genotypes.
Characterizing neutralizing antibody epitopes and their role in viral entry inhibition.
Assessing adjuvant mechanisms and their impact on the strength and duration of immune response.
Investigating cross-type protection between phylogenetically related HPV strains.
Modeling human immune responses using preclinical animal systems or in vitro assays.
The 9-valent vaccine structure has also become a cornerstone for research into VLP vaccine technology, allowing scientists to compare recombinant antigen designs, purification methods, and adjuvant formulations that enhance immune recognition.
Its application extends to molecular studies on capsid protein folding, assembly stability, and receptor-binding mechanisms critical to HPV infectivity.
In preclinical immunology laboratories, this vaccine is commonly used to evaluate neutralizing antibody titers, cytokine responses, and germinal center B-cell dynamics. By comparing immune kinetics against monovalent and quadrivalent formulations, researchers gain insights into the optimization of next-generation, broad-spectrum HPV vaccine candidates.
Research Significance
The 9-Valent HPV Vaccine facilitates research into:
Multivalent vaccine development for viral pathogens.
Immunodominance and antigen competition within complex formulations.
Mucosal immunity mechanisms related to HPV infection sites.
Long-term antibody persistence and memory B-cell activation.
Adjuvant comparative studies, particularly alum- or AS04-based systems.
The recombinant expression system provides consistent, high-yield VLP production with low endotoxin content, ensuring reproducibility in immunogenicity experiments. For laboratories focusing on vaccine design, structural virology, or comparative immunology, the 9-Valent HPV Vaccine serves as an essential control and reference model.
Product Specifications
| Item | Details |
|---|---|
| Product Name | 9-Valent Human Papillomavirus (HPV) Vaccine |
| Synonyms | HPV9 Vaccine, Nonavalent HPV Vaccine, 9vHPV |
| CAS Number | — |
| Composition | Recombinant L1 virus-like particles (VLPs) from HPV types 6, 11, 16, 18, 31, 33, 45, 52, 58 |
| Molecular Type | Recombinant protein-based vaccine |
| Expression System | Yeast (Saccharomyces cerevisiae) |
| Formulation | Liquid or lyophilized form, adjuvanted with aluminum hydroxyphosphate sulfate |
| Purity | ≥99% |
| Appearance | Clear to slightly opalescent suspension |
| Storage | 2–8 °C, protected from light |
| Applications | Vaccine immunology, HPV antigenicity research, preclinical vaccine evaluation |
| Category | Recombinant VLP vaccine |
| Mechanistic Focus | Induction of neutralizing antibodies against HPV L1 capsid antigens |
| Supplier Type | Laboratory and preclinical research reagent provider |
| Intended Use | For laboratory and preclinical research only |
Mechanism of Action
The 9-Valent HPV Vaccine works by mimicking the structure of the native HPV virion through its recombinant L1 capsid proteins, which self-assemble into virus-like particles (VLPs). These VLPs present the same conformational epitopes found on natural HPV, stimulating neutralizing antibody production without containing viral genetic material.
1. Antigenic Presentation
The VLPs in the vaccine expose conformational epitopes that are recognized by the immune system as authentic viral structures. This induces a robust B-cell–mediated immune response, leading to the generation of high-affinity neutralizing antibodies.
2. Activation of Adaptive Immunity
Upon administration in research models, the vaccine antigens are taken up by antigen-presenting cells (APCs), which process and present them to helper T-cells (CD4+). This promotes cytokine release and stimulates B-cell maturation and antibody isotype switching.
3. Cross-Type Protection Mechanism
Although each HPV type is represented by a distinct L1 VLP, the 9-valent formulation elicits cross-neutralizing antibodies due to partial epitope homology among related HPV genotypes, such as HPV16/31 and HPV18/45 clusters. This enables the exploration of broad-spectrum immunogenicity in laboratory assays.
4. Long-Term Immune Memory
Experimental data indicate that the vaccine triggers durable immune memory characterized by sustained high antibody titers and persistent germinal center activity. These features make it a valuable model for studying immune longevity and booster response mechanisms.
5. Comparative Immunogenicity Studies
Researchers often compare the 9-valent formulation with quadrivalent or bivalent HPV vaccines to evaluate antigenic competition, synergistic immune responses, and dose optimization. The inclusion of nine HPV L1 antigens allows detailed study of multivalent vaccine complexity.
Side Effects
For laboratory and animal research contexts, the following considerations are noted:
Minor local inflammatory reactions at the site of injection in model organisms.
Mild fever or cytokine activation observed in immunized animals.
No evidence of viral infection, as VLPs contain no DNA.
Ensure all handling follows biosafety level 1 (BSL-1) standards.
Avoid repeated freeze-thaw cycles to maintain VLP integrity.
Keywords
9-valent HPV vaccine, recombinant VLP, HPV L1 antigen, HPV immunogenicity, neutralizing antibody research, virus-like particle vaccine, HPV prevention research, multivalent vaccine development, high-purity HPV reagent, laboratory vaccine study
Shipping Guarantee
All shipments are handled using validated cold-chain logistics to preserve peptide and protein integrity. Each package is sealed in moisture-proof containers with temperature monitoring and shipped via express international couriers with full tracking.
Trade Assurance
We guarantee product authenticity, verified ≥99% purity, and analytical validation by HPLC and protein integrity assays. A Certificate of Analysis (CoA) is included with each batch. Replacement or refund applies if any deviation occurs.
Payment Support
We provide flexible international payment options: PayPal, major credit cards, telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are encrypted and verified to ensure data and fund security.
Disclaimer
All products are intended for laboratory research use only. They are not vaccines for human or veterinary administration. No medical, diagnostic, or therapeutic claims are implied. Researchers must follow biosafety and institutional research regulations.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
1. What is the 9-Valent HPV Vaccine used for in research?
It is used to study immunogenicity, antibody formation, and VLP-based vaccine mechanisms in HPV research.
2. Which HPV types does it cover?
It targets HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
3. Is the 9-Valent HPV Vaccine infectious?
No. It contains no viral DNA and cannot cause infection.
4. What expression system is used for its production?
A recombinant yeast-based (Saccharomyces cerevisiae) system.
5. How should the vaccine be stored?
Store at 2–8°C and avoid repeated freeze–thaw cycles.
6. Can this product be used in human vaccination?
No. It is for laboratory and preclinical research only.
7. Does it induce both humoral and cellular responses?
Yes. It primarily induces neutralizing antibodies but also supports helper T-cell activation.
8. What are the main research applications?
HPV immunology, VLP structure studies, and adjuvant mechanism analysis.
9. Is a CoA included?
Yes. Each batch comes with a Certificate of Analysis verifying purity and structure.
10. Can it be used for comparative vaccine design studies?
Yes, it is widely used to evaluate multivalent antigen behavior.
11. Does it require adjuvant addition in experiments?
It can be studied with or without alum-based adjuvants depending on experimental design.
12. What biosafety level is required?
BSL-1 is sufficient since it contains no infectious material.
13. Is it suitable for neutralization assays?
Yes, it serves as an antigenic reference for HPV antibody assays.
14. What makes it different from the quadrivalent HPV vaccine?
It includes five additional HPV types for broader research coverage.
15. What is its purity specification?
≥99%, validated by HPLC and protein characterization assays.












Reviews
There are no reviews yet.