No products in the cart.
Sale
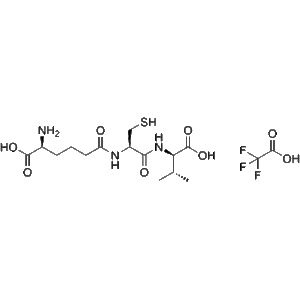
ACV Tripeptide TFA – High Purity Biosynthetic Precursor of ?-Lactam Antibiotics – GMP Peptide
Original price was: $18.00.$16.00Current price is: $16.00.
ACV Tripeptide TFA (GMP-grade) is the trifluoroacetate form of the ACV tripeptide (?-L-?-aminoadipyl-L-cysteinyl-D-valine), a key non-ribosomal peptide precursor in penicillin and cephalosporin production. Useful in antibiotic biosynthesis and NRPS research. For laboratory research use only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
ACV Tripeptide TFA is the trifluoroacetate (TFA) salt of ?-L-?-aminoadipyl-L-cysteinyl-D-valine (ACV), a tripeptide biosynthesized by ACV synthase during the production of penicillin and cephalosporin antibiotics. This compound, produced under strict GMP conditions, offers researchers a high-purity, stable form for experimental models spanning enzyme kinetics, synthetic biology, and antibiotic biosynthesis research.
ACV, first identified in Penicillium chrysogenum and Acremonium chrysogenum, serves as the critical starting tri-amino acid substrate in the ?-lactam biosynthetic pathway. The multifunctional, non-ribosomal enzyme ACV synthetase catalyzes sequential activation, condensation, and epimerization of ?-aminoadipate, cysteine, and valine to form ACV. This step is regarded as the rate-limiting and regulatory entry point into the antibiotic assembly line, making ACV analogs highly relevant for biochemical studies and metabolic engineering.
By supplying the TFA salt of ACV, this product enhances solubility and stability, facilitating handling in various assay environments such as enzyme reconstitution, synthetic biology platforms, and precursor feeding experiments. ACV Tripeptide TFA is an indispensable tool for exploring non-ribosomal peptide synthesis mechanisms, optimizing ?-lactam yields, or engineering novel derivatives with improved antimicrobial profiles.
Quality assurance includes rigorous HPLC purity testing to confirm structural integrity, as well as consistency across batches—crucial for reproducible results in sensitive biosynthetic applications.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | ACV Tripeptide TFA |
| Synonyms | ?-L-?-Aminoadipyl-L-Cysteinyl-D-Valine TFA; ACV precursor peptide |
| CAS No. | (Not assigned for TFA form) |
| Molecular Formula | C??H??F?N?O?S (TFA salt; parent tripeptide core) |
| Molecular Weight | ~477.45 g/mol (TFA form) |
| Purity | ?98–99% (HPLC grade) |
| Form | Lyophilized powder, TFA salt |
| Appearance | White to off-white solid |
| Storage | –80 °C for long-term; –20 °C for short-term (powder form, moisture-protected) |
| Solubility | Soluble in water and DMSO, suitable for biosynthetic assays |
| Manufacturing Standard | GMP-compliant peptide synthesis |
| Applications | Non-ribosomal peptide synthesis, antibiotic precursor studies, enzymology assays |
| Regulatory Status | For laboratory research use only; not for clinical or veterinary purposes |
Mechanism of Action & Research Applications
ACV Tripeptide TFA operates as the immediate biosynthetic precursor to ?-lactam antibiotics. The molecule, ?-L-?-aminoadipyl-L-cysteinyl-D-valine, is synthesized by ACV synthetase, a massive non-ribosomal peptide synthetase (NRPS) that catalyzes formation of ACV through ATP-dependent activation, peptide bond formation, and epimerization. It offers a gateway into classic ?-lactam generation—first converted to isopenicillin N via isopenicillin N synthase, then further transformed into diverse penicillins and cephalosporins.
By supplying ACV directly, researchers bypass rate-limiting steps in penicillin biosynthesis. This enables controlled feeding experiments in producing organisms, streamlined in vitro enzymatic assays, and enhanced productivity in metabolic engineering of antibiotic pathways. Due to the TFA form’s improved solubility and stability, ACV Tripeptide TFA is optimal for probing enzymatic kinetics, substrate tolerances, mechanistic studies, and NRPS domain functions.
Applications include:
Enzyme kinetics and mechanistic assays of ACV synthetase and downstream tailoring enzymes.
Precursor feeding approaches in penicillin- or cephalosporin-producing cell cultures to increase yield.
Engineering of modified ?-lactam backbones by introducing analogs in place of ACV.
Synthetic biology research exploring redesign of NRPS modules.
Biotechnological production of novel antibiotic derivatives.
Because ACV formation consumes multiple ATP per peptide, its addition provides a convenient handle to study the energetic burden and regulation of ?-lactam biosynthesis curves in native and heterologous systems.
Development & Formulation Notes
ACV Tripeptide TFA has been adopted due to its chemical stability and solubility advantages. The trifluoroacetate (TFA) salt enhances handling characteristics compared to the free acid. ACV Tripeptide is inherently sensitive to hydrolysis and enzymatic degradation; TFA salt mitigates this by buffering the tripeptide environment and prolonging shelf life—reported storage stability spans over a year at –20 °C, and up to two years at –80 °C in lyophilized form.
For formulation, ACV Tripeptide TFA should be dissolved in DMSO or water-based buffers shortly before use. Concentrated stock solutions (e.g., 10–50 mM) stored at low temperatures help maintain integrity and minimize degradation. Use can involve direct feeding to microorganisms, enzyme incubation assays, or incorporation into bioreactor media for production experiments.
Given its role at the nexus of NRPS-triggered biosynthesis, ACV serves in enzyme mechanism dissection—including substrate specificity, domain interactions, and catalytic efficiency. Researchers should take extra care to include control reactions and verify product formation using HPLC-MS or specific ?-lactam detection.
A batch of this peptide has been synthesized under GMP compliance, accompanied by a detailed Certificate of Analysis documenting purity, identity (MS), and physico-chemical characteristics, ensuring consistency for experimental reproducibility.
Keywords
ACV Tripeptide TFA; delta-L-aminoadipyl-L-cysteinyl-D-valine; ?-lactam precursor; ACV synthase; non-ribosomal peptide synthetase; penicillin biosynthesis; cephalosporin precursor; GMP peptide; antibiotic biosynthesis research; research-use-only.
Disclaimer
ACV Tripeptide TFA is supplied strictly for laboratory research use only. It is not a pharmaceutical, diagnostic, or veterinary product and is not intended for human ingestion, injection, or clinical use. Handle according to institutional biosafety guidelines, use appropriate PPE, and dispose of all materials according to local regulations. Users are solely responsible for compliance with laws and regulations applicable to their laboratory.
Packaging & Availability
Standard Packs: 1?mg, 5?mg, 10?mg lyophilized powder
Bulk/Wholesale: Multi-gram lots with batch reservation and scheduled delivery options
Documentation: CoA, HPLC trace, MS identity included; additional QC documentation available upon request
Quality Assurance & Logistics
Manufactured under GMP-compliant conditions with validated peptide synthesis and purification methods
Routine testing includes identity (MS), purity (HPLC), residual solvents, and appearance
Optional endotoxin and bioburden testing available
Packaged to protect from moisture, light, and temperature excursions during transport
Recommended shipping: cold-chain or desiccant-protected dry shipment
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 86 × 25 × 86 cm |













Thomos –
We are satisfied with product and service .