No products in the cart.
Sale
Anastrozole | CAS 120511-73-1 | Non-Steroidal Aromatase Inhibitor
Original price was: $3.00.$2.00Current price is: $2.00.
Anastrozole (CAS 120511-73-1) is a non-steroidal aromatase inhibitor widely used in laboratory research to investigate estrogen biosynthesis inhibition and endocrine modulation. It is commonly applied in studies of hormone-dependent cancer models, enzyme kinetics, and estrogen receptor signaling. Its potent, selective inhibition of aromatase makes it a valuable tool for mechanistic studies in preclinical research.
Description
Product Description
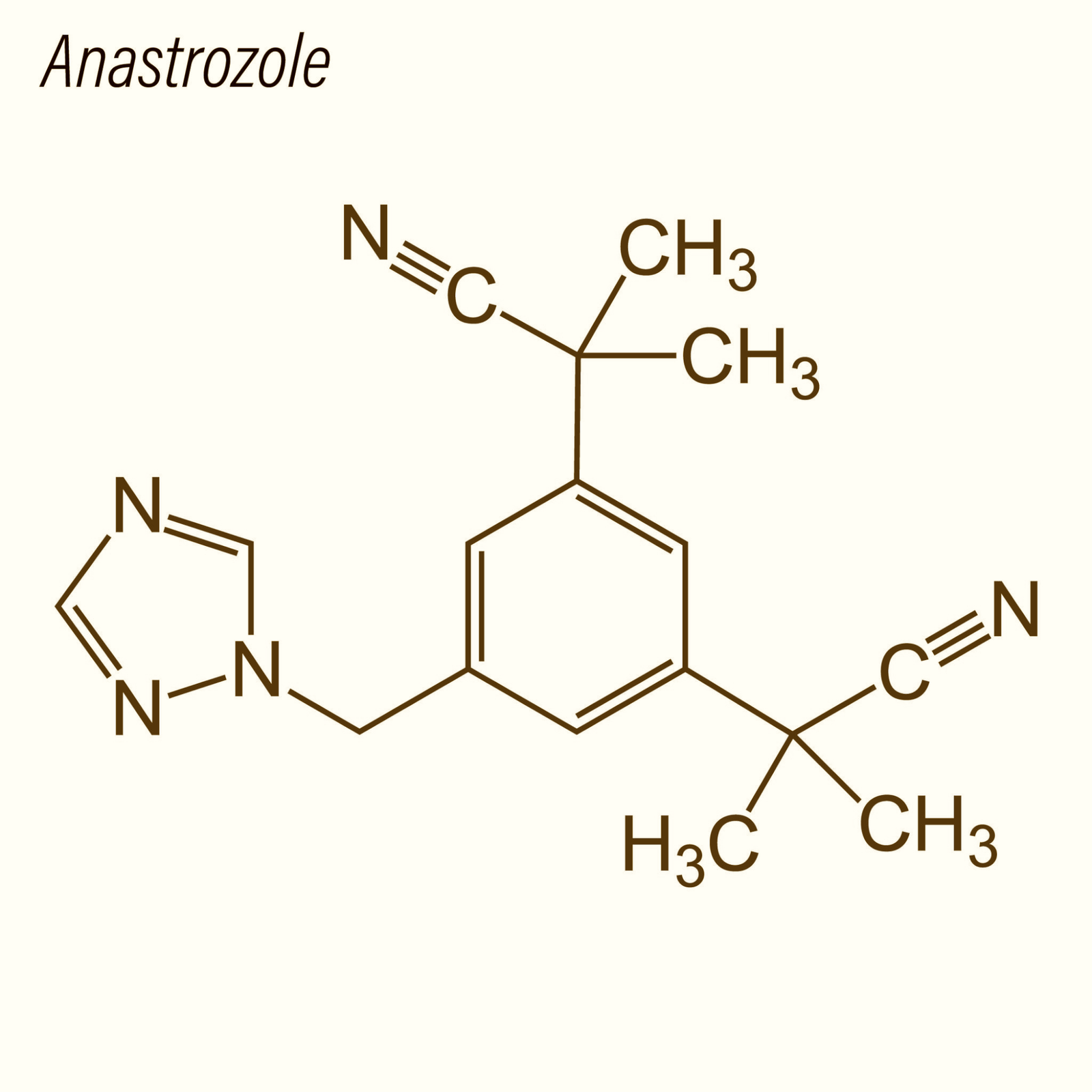
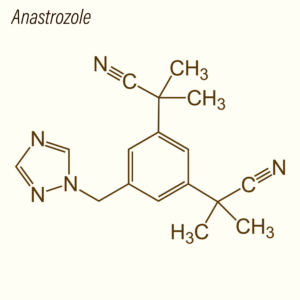
Anastrozole is a synthetic triazole derivative that functions as a selective, reversible inhibitor of the aromatase enzyme (CYP19A1), which catalyzes the conversion of androgens to estrogens. Unlike steroidal aromatase inhibitors, anastrozole does not irreversibly bind to the enzyme but competitively inhibits its activity, providing a model for studying reversible enzyme inhibition and hormone regulation.
In preclinical research, anastrozole is employed to explore:
The molecular mechanisms of estrogen synthesis inhibition.
Effects on estrogen-dependent cell proliferation and gene expression.
Comparative studies of steroidal versus non-steroidal aromatase inhibitors.
Pharmacokinetic modeling of reversible enzyme inhibition.
Structurally, anastrozole’s triazole ring interacts with the heme group of aromatase, preventing substrate binding. This selective inhibition reduces estradiol and estrone production in model systems, enabling researchers to study estrogen-sensitive cellular processes, transcriptional regulation, and endocrine feedback loops.
Anastrozole is also utilized in combination studies with other biochemical agents to evaluate synergistic effects on hormone metabolism, cell cycle regulation, and signal transduction pathways. Its high specificity and minimal off-target activity make it a standard reference compound in laboratory studies of estrogen biosynthesis and endocrine disruption.
Product Specifications
| Item | Specification |
|---|---|
| Product Name | Anastrozole |
| CAS Number | 120511-73-1 |
| Synonyms | Arimidex; 1,2,4-Triazole derivative |
| Molecular Formula | C17H19N5 |
| Molecular Weight | 293.37 g/mol |
| Purity | ≥99% |
| Appearance | White to off-white crystalline powder |
| Solubility | Soluble in DMSO, ethanol, and methanol; poorly soluble in water |
| Storage Temperature | 2–8 °C |
| Category | Non-steroidal aromatase inhibitor; research chemical |
| Applications | Endocrine research, estrogen suppression studies, hormone-dependent cell modeling |
| Formulation | Suitable for in vitro and ex vivo research applications |
| Stability | Stable under recommended storage conditions |
| Shelf Life | 24 months |
| Supplier Type | Research chemical supplier |
| Intended Use | For laboratory research use only |
Mechanism of Action
Anastrozole functions as a reversible, non-steroidal inhibitor of aromatase (CYP19A1). By binding competitively to the heme iron within the enzyme, it prevents the conversion of androgens (testosterone and androstenedione) into estrogens (estradiol and estrone). This reduces estrogen availability in endocrine-sensitive systems.
1. Aromatase Inhibition
Anastrozole’s triazole moiety chelates the heme iron in aromatase, inhibiting catalytic activity without covalent modification. This reversible binding allows researchers to study time- and concentration-dependent enzyme inhibition.
2. Downstream Molecular Effects
Reduction in estrogen synthesis leads to decreased activation of estrogen receptors (ERα, ERβ) in target cells. This affects transcriptional regulation of estrogen-responsive genes involved in cell proliferation, apoptosis, and differentiation.
3. Experimental Applications
Modeling hormone-dependent cancer growth.
Investigating feedback loops in endocrine regulation.
Comparative analysis of non-steroidal versus steroidal aromatase inhibitors.
Evaluating effects on estrogen-mediated transcription and cell signaling in vitro.

Side Effects
At research concentrations, anastrozole is generally well-tolerated in laboratory models. Possible effects include:
Dose-dependent inhibition of estrogen-sensitive cell proliferation.
Modulation of hormone-responsive gene expression.
Potential oxidative stress in sensitive cell lines at high concentrations.
These effects are strictly experimental and observed under controlled laboratory conditions. The compound is not intended for human or veterinary use.
Keywords
Anastrozole, CAS 120511-73-1, non-steroidal aromatase inhibitor, CYP19A1 research, estrogen biosynthesis inhibition, endocrine research chemical, hormone-sensitive cancer model, reversible enzyme inhibitor, high-purity research compound, research chemical manufacturer, OEM & bulk production China.
Shipping Guarantee
All Anastrozole shipments are handled using validated cold-chain logistics to preserve compound integrity. Each package is sealed in moisture-proof containers with secondary protective wrapping and continuous temperature monitoring. Products are shipped via express international couriers with full tracking and insurance coverage.
Trade Assurance
We Anastrozole ensure product authenticity, verified ≥99% purity, and compliance with analytical standards (HPLC, MS, and NMR). Each batch is supplied with a Certificate of Analysis (CoA). Our trade assurance policy guarantees replacement or refund for any deviation from listed specifications.
Payment Support
We Anastrozole provide flexible and secure global payment options to support international research transactions. Accepted payment methods include PayPal, major credit cards (Visa, MasterCard, American Express), telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are protected by industry-standard encryption and verified payment gateways to ensure confidentiality and fund security.
Disclaimer
All Anastrozole products listed are intended for laboratory research use only and not for human or veterinary use. They are not drugs, medical devices, or diagnostics and should not be administered to humans or animals. Researchers must handle all materials in accordance with institutional biosafety and chemical safety guidelines. The information provided is for scientific reference only and does not imply therapeutic efficacy, safety, or regulatory approval.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
Is Anastrozole a peptide?
No, it is a small-molecule, non-steroidal aromatase inhibitor.
What is Anastrozole used for in research?
It is used to study estrogen biosynthesis inhibition, hormone-dependent cell signaling, and endocrine regulation in preclinical models.
What purity level is offered?
≥99%, verified by HPLC and MS.
How should Anastrozole be stored?
Store at 2–8 °C, protected from light and moisture.
Can it be used in combination with other research compounds?
Yes, commonly used with hormone receptor modulators and other enzymatic inhibitors.
Is it available for bulk or OEM production?
Yes, OEM and bulk production for research purposes are available.
Does it come with a CoA?
Yes, each batch is accompanied by a Certificate of Analysis confirming purity and identity.
Is Anastrozole soluble in water?
Poorly soluble in water; soluble in DMSO, ethanol, and methanol.
Can international researchers order directly?
Yes, direct orders are supported globally with full shipping documentation.
What is the main mechanism of action?
Reversible competitive inhibition of aromatase, reducing estrogen synthesis in model systems.
Is this suitable for cell culture studies?
Yes, it is widely applied in vitro for studying hormone-sensitive cells.
Are there any observed lab-side effects?
Dose-dependent reduction in proliferation of estrogen-sensitive cells; effects are strictly experimental.
Why choose your supplier for Anastrozole?
Our facility is a factory peptide and small-molecule supplier providing high-purity compounds, OEM & bulk production from China.
Does it interfere with other P450 enzymes?
Minimal off-target effects, high selectivity for CYP19A1 aromatase.
Can it be purchased online?
Yes, orders can be placed through our online platform with secure payment and full trade assurance.














Reviews
There are no reviews yet.