Anastrozole Tablets 28mg Factory wholesale
$2.00
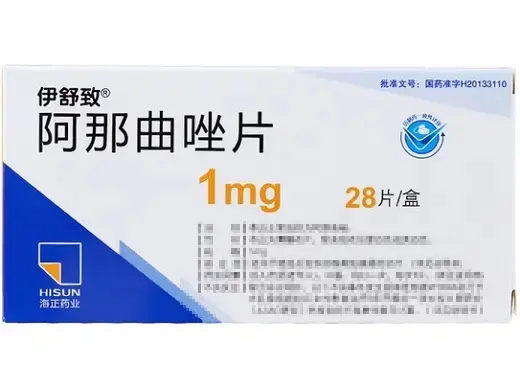
Anastrozole (brand: Yishuzhi®) is a potent third-generation non-steroidal aromatase inhibitor used primarily in research focused on estrogen-dependent breast cancer and hormone-related pathways. Each box contains 28 film-coated tablets (1?mg each). Manufactured by Zhejiang Hisun Pharmaceutical Co., Ltd., approved under China NMPA H20133110 (product code 86904641003065; barcode 6939030230917).
?? For laboratory research use only.?Please consult the staff for other purposes?
描述
Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Anastrozole Tablets (Yishuzhi®) |
| Generic Name | Anastrozole |
| CAS Number | 120511?73?1 :contentReference[oaicite:5]{index=5} |
| Molecular Formula | C??H??N? :contentReference[oaicite:6]{index=6} |
| Molecular Weight | 293.37?g/mol :contentReference[oaicite:7]{index=7} |
| Dosage Form | Film-coated oral tablet |
| Strength | 1?mg per tablet |
| Pack Size | 28 tablets per box |
| Approval Number | H20133110 (China NMPA) |
| Product Code | 86904641003065 |
| Manufacturer | Zhejiang Hisun Pharmaceutical Co., Ltd. |
| Barcode | 6939030230917 |
| Storage | Store at room temperature; protect from moisture |
| Intended Use | Laboratory research only |
Mechanism of Action
Anastrozole reversibly inhibits aromatase (CYP19), blocking conversion of androgens to estrogens, resulting in ?96% aromatase inhibition at 1?mg/day and an ~85% reduction in circulating estradiol news.qq.com+14en.wikipedia.org+14go.drugbank.com+14.
Research Applications & Pharmacology
-
Breast cancer research: hormone-dependent growth, estrogen receptor modulation
-
Endocrine pathway studies: estrogen synthesis, aromatase activity assays
-
Pharmacokinetics: Protein binding ~40%, hepatic metabolism (~85%), t½ 40–50?hrs, renal excretion <11%
Safety Profile & Common Adverse Effects
-
Clinical observations: hot flashes, fatigue, joint pain, nausea, headache, bone mineral density loss, mood disturbances, elevated liver enzymes
-
Handling notes: wear PPE, store tablets properly, report signs of hormonal modulation in animal models
-
Contraindications: not for pregnancy, premenopausal uterine bleeding, severe hepatic/renal impairment
?? Research?Use Disclaimer
This product is exclusively for laboratory research use and is not approved for therapeutic, diagnostic, clinical, or veterinary use. Misuse may pose safety risks and invalidate research findings.
其他信息
| 重量 | 1.1 公斤 |
|---|---|
| 尺寸 | 18 × 16 × 18 厘米 |








评价
目前还没有评价