Anrikefon Acetate (HSK21542 Acetate) – GMP Manufacturer
$16.00
Anrikefon acetate (HSK21542 acetate) is a high-purity, peripherally restricted kappa-opioid receptor agonist with potent analgesic efficacy and reduced central nervous system side effects. GMP-grade, purity ? 99%, suitable for wholesale and retail. For laboratory research use only.?For wholesale prices, other specifications and uses, please consult our staff?
描述
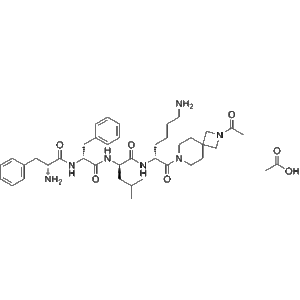
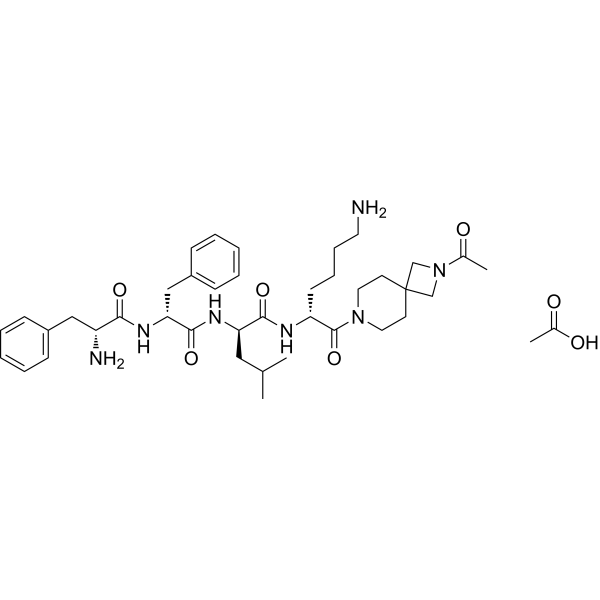
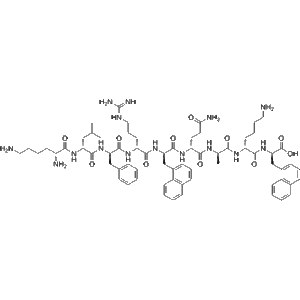
Anrikefon acetate (CAS 2584931-05-3), also known as HSK21542 acetate, is a potent and selective agonist of the kappa-opioid receptor (KOR), engineered to exert strong analgesic effects while being peripherally restricted to minimize central nervous system (CNS) liabilities.
Preclinical studies demonstrate that Anrikefon acetate robustly inhibits pain-related behaviors in visceral, inflammatory, and neuropathic animal models, showing comparable or superior efficacy to CR845 but without CNS effects such as sedation, dysphoria, or respiratory depression. In vitro, it binds KOR with high affinity (IC?? ? 0.54 nM; K_d ? 0.068 nM) and potently inhibits cAMP production (EC?? ? 2.41 pM), underscoring exceptional receptor selectivity and activation potency.
Manufactured under GMP-compliant processes, this lyophilized peptide offers high purity and consistent quality for dependable research use.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Anrikefon Acetate (HSK21542 Acetate) |
| Synonyms | HSK21542 acetate; Anrikefon acetate |
| CAS No. | 2584931-05-3 |
| Chemical Identity | Peripherally restricted KOR agonist peptide |
| Purity | ? 99% (HPLC, GMP-grade) |
| Form | Lyophilized powder |
| Appearance | White to off-white solid |
| Molecular Formula | C??H??N?O? |
| Molecular Weight | ~763.97 g/mol |
| Solubility | Soluble in DMSO (up to ~10 mM); other solvents per lab SOPs ProbeChem |
| Stability/Storage | Store at ?20 °C, desiccated and light-protected; avoid freeze–thaw |
| Endotoxin | Research grade; see CoA |
| Manufacturing Standard | GMP-compliant synthesis and purification |
| Applications | Pain and analgesia research; KOR signaling studies |
| Regulatory Status | For laboratory research use only. Not for human or veterinary use |
Mechanism of Action & Research Applications
Mechanism of Action
Anrikefon acetate is a peripherally restricted KOR agonist that binds with high affinity and activates KOR signaling, leading to downstream G-protein mediated inhibition of neuronal excitability—resulting in potent analgesic effects without CNS-related side effects.
Research Applications
-
Pain Modeling: Proven efficacy in animal models of visceral, incisional, and neuropathic pain; effective reversal by KOR antagonists confirms mechanistic specificity.
-
Comparative Studies: Serves as a benchmark against other peripheral KOR agonists like CR845 (difelikefalin) for comparative pharmacology.
-
CNS Safety Profiling: Use in models requiring analgesia without sedation or respiratory compromise, thanks to limited CNS penetration.
-
Receptor Pharmacodynamics: Excellent tool for dissecting peripheral KOR-mediated signaling mechanisms due to its high potency and selectivity.
Development & Formulation Notes
Stock Solutions & Handling
Prepare concentrated stocks (~1–10 mM) in DMSO. Avoid repeated freeze–thaw by aliquoting. For aqueous use, dilute freshly before assays and consider adding carrier proteins per SOP.
Assay Design
Run dose–response experiments in relevant in vitro and in vivo models. Use antagonists to confirm KOR-specific effects and monitor markers of sensory neuron activation.
Combination & Pathway Mapping
Combine with NSAIDs or peripheral analgesics to evaluate potential additive effects. Include behavioral and molecular readouts to characterize comprehensive analgesic profiles.
Disclaimer
Anrikefon acetate is provided strictly for laboratory research purposes only. It is neither a pharmaceutical nor a diagnostic agent and is not approved for human or veterinary use. Handle under institutional biosafety guidelines using appropriate PPE. Dispose of waste responsibly. All data referenced are from preclinical studies; users are responsible for ensuring suitability and regulatory compliance.
Keywords
Anrikefon acetate; HSK21542 acetate; peripheral KOR agonist; kappa-opioid receptor agonist; analgesic peptide; potent analgesia without CNS side effects; GMP peptide; CAS 2584931-05-3; research-use-only analgesic.
Packaging & Availability
-
Standard Packs: 1 mg, 5 mg, 10 mg (custom packs upon request)
-
Bulk/Wholesale: Multi-gram quantities with batch reservation and scheduled delivery options
-
Documentation: Certificate of Analysis (CoA), HPLC trace, and MS identity included; extended QC available for audits
Quality Assurance
GMP-grade synthesis with batch testing for identity (MS), purity (HPLC), moisture, and appearance. Stability and solubility recommendations accompany each lot; endotoxin data provided upon request.
Custom Services
-
Custom aliquoting and labeling compatible with LIMS/ELN systems
-
Formulation support for solvent systems and carrier selection
-
Stability study design for shipping and storage scenarios
-
Experimental protocol planning for peripheral analgesia models
其他信息
| 重量 | 0.6 公斤 |
|---|---|
| 尺寸 | 58 × 42 × 58 厘米 |








评价
目前还没有评价