No products in the cart.
Sale
ASB20123 | GMP Supplier & Manufacturer for Research Use
Original price was: $26.00.$20.00Current price is: $20.00.
ASB20123 is a synthetic CNP/ghrelin chimeric peptide designed to promote bone growth and support research into growth disorders, dwarfism, and skeletal development. Produced under GMP-grade standards, ASB20123 is suitable for in vitro and in vivo studies, offering reproducible results for laboratories focusing on endocrine and skeletal research.
Description
1. Product Description
ASB20123 is a chimeric peptide combining C-type natriuretic peptide (CNP) and ghrelin sequences, engineered to stimulate longitudinal bone growth through synergistic effects on growth plate chondrocytes and bone remodeling pathways. Growth plate research is critical for understanding disorders such as achondroplasia, dwarfism, and other skeletal dysplasias.
Background
CNP plays a vital role in endochondral ossification, regulating chondrocyte proliferation and differentiation. Ghrelin, a growth hormone secretagogue, stimulates GH release and promotes anabolic effects on skeletal tissue. ASB20123 integrates these two mechanisms, creating a potent bone growth-promoting peptide for preclinical research applications.
Scientific Relevance
Bone Growth Research: Enables studies on chondrocyte proliferation, differentiation, and bone elongation.
Endocrine Pathway Analysis: Facilitates investigation of CNP/NPR-B signaling and ghrelin-GH axis interaction.
Therapeutic Development: Provides insights for growth disorder therapeutics, including pharmacological interventions in dwarfism.
Comparative Peptide Studies: Useful for testing different analogues and dosing strategies for skeletal growth optimization.
Comparison with Other Peptides
CNP analogues – Primarily target growth plate chondrocytes.
Ghrelin analogues – Mainly enhance GH secretion.
ASB20123 – Combines both CNP and ghrelin functions, offering additive or synergistic effects on bone growth.
Advantages in Research
Dual mechanism ensures enhanced bone growth stimulation.
GMP-grade production guarantees reproducible results in laboratory experiments.
Compatible with in vitro chondrocyte cultures, ex vivo bone models, and in vivo animal studies.
Stable lyophilized form allows long-term storage and transport.
2. Product Specifications
| Parameter | Details |
|---|---|
| Product Name | ASB20123 |
| Synonyms | CNP/Ghrelin chimeric peptide |
| CAS Number | N/A (research peptide) |
| Molecular Type | Synthetic chimeric peptide |
| Molecular Formula | Peptide sequence-specific (CNP + Ghrelin hybrid) |
| Molecular Weight | ~4000–5000 Da (depending on peptide length) |
| Appearance | White to off-white lyophilized powder |
| Purity | 98% (HPLC) |
| Solubility | Soluble in sterile aqueous buffers, DMSO |
| Stability | Stable 24 months in lyophilized form |
| Storage Conditions | Store at -20°C; protect from light and moisture; avoid repeated freeze-thaw |
| Mechanism | Stimulates bone growth via CNP/NPR-B signaling and ghrelin-GH axis |
| Applications | Growth disorder research, dwarfism models, skeletal development studies |
| GMP Compliance | Yes, manufactured under GMP standards |
| Availability | Wholesale & retail supply |
| Experimental Models | Rodent growth plate studies, in vitro chondrocytes, ex vivo bone cultures |
| Safety Considerations | For research use only; not for human or veterinary administration |
3. Mechanism of Action & Research Applications
Mechanism of Action
ASB20123 functions via dual signaling pathways:
CNP Component
Activates natriuretic peptide receptor-B (NPR-B) on chondrocytes.
Increases intracellular cGMP levels, promoting chondrocyte proliferation and differentiation.
Enhances endochondral ossification, accelerating longitudinal bone growth.
Ghrelin Component
Stimulates growth hormone (GH) release via hypothalamic-pituitary axis.
Induces IGF-1 secretion, which synergistically supports bone formation.
Research Applications
Growth Disorder Models
Investigating therapies for achondroplasia, dwarfism, and other skeletal dysplasias.
Endocrinology Studies
Exploring CNP/NPR-B signaling, ghrelin-GH axis interactions, and growth factor regulation.
In Vitro Chondrocyte Studies
Assessing cell proliferation, differentiation, and extracellular matrix deposition in cultured growth plate cells.
Ex Vivo Bone Culture
Monitoring epiphyseal growth plate elongation and bone matrix formation.
Animal Models
Testing pharmacological efficacy in rodent or larger animal models of growth deficiency.
Translational Research
Informing development of novel therapeutics for pediatric growth disorders.
Advantages for Research
Synergistic effect improves bone growth stimulation over single-component peptides.
GMP-grade reproducibility ensures consistency across preclinical studies.
Versatility for multi-modal applications in chondrocyte biology, endocrinology, and skeletal research.

4. Side Effects (Research Reference)
In preclinical studies, ASB20123 demonstrates:
Dose-Dependent Growth Stimulation: Excessive dosing may lead to overgrowth in animal models.
Metabolic Effects: Ghrelin component may influence appetite and glucose metabolism.
Skeletal Abnormalities: High doses over prolonged periods may alter bone geometry.
Species-Specific Responses: Rodent vs. larger animal responses may vary.
Stability Considerations: Proper storage essential to maintain peptide activity.
These observations are for laboratory research reference. ASB20123 is not approved for human or veterinary use.
5. Disclaimer
For laboratory research use only. Not for human or veterinary use.
6. Keywords
ASB20123
CNP/Ghrelin chimeric peptide
Bone growth research peptide
Growth disorder research peptide
Dwarfism research peptide
GMP supplier ASB20123
Skeletal development peptide
Growth plate research peptide
Laboratory use bone growth peptide
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |
2 reviews for ASB20123 | GMP Supplier & Manufacturer for Research Use
What is ASB20123?
A CNP/ghrelin chimeric peptide that stimulates bone growth.
What are its research applications?
Growth disorders, dwarfism, skeletal development studies.
What signaling pathways does it target?
CNP/NPR-B signaling and ghrelin-GH axis.
Is ASB20123 GMP-grade?
Yes, manufactured under GMP standards.


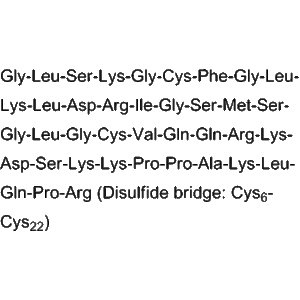














papasmur –
The supplier responded quickly and handled the order professionally.
Dalion –
amazing logistics speed