No products in the cart.
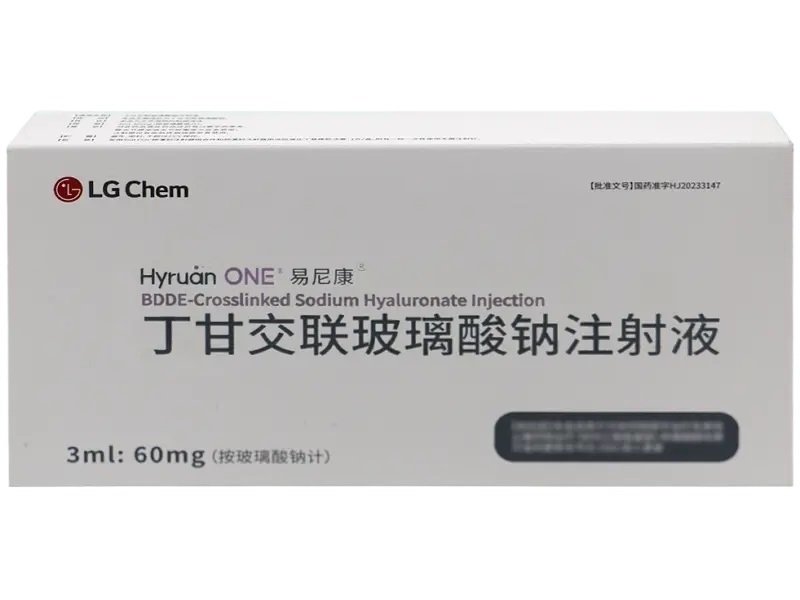
BDDE-Cross Linked Sodium Hyaluronate Injection – 60mg Prefilled Syringe
$1.00
BDDE-Cross Linked Sodium Hyaluronate Injection (3ml:60mg) in a prefilled syringe with an attached needle. Approved under HJ20233147 and manufactured by LG Chem, Ltd. (Korea). Designed for in vitro and in vivo laboratory research. Supports both bulk wholesale and small quantity retail.?Please consult staff for price, other specifications and uses?
Description
BDDE-Cross Linked Sodium Hyaluronate is a chemically stabilized hyaluronic acid designed to enhance bio-stability and viscoelastic properties. This product is widely used in joint lubrication, cartilage protection, and soft tissue regeneration research.
The formulation employs 1,4-butanediol diglycidyl ether (BDDE) as a crosslinker, creating a three-dimensional network that resists enzymatic degradation. It mimics synovial fluid to improve mobility and comfort in experimental osteoarthritis models.
This injection comes in a prefilled syringe (3ml:60mg) with a sterile needle, sealed for safety and precision. Manufactured by LG Chem, Ltd. (South Korea) and registered under China NMPA HJ20233147, product code 86981788000164.
?? Strictly for laboratory research use only. Not intended for human or animal clinical application.
BDDE-Cross Linked Sodium Hyaluronate Injection Product Specifications
| Parameter | Details |
|---|---|
| Product Name | BDDE-Cross Linked Sodium Hyaluronate Injection |
| Generic Name | Sodium Hyaluronate (crosslinked with BDDE) |
| CAS Number | 9067-32-7 (Hyaluronic Acid) |
| Molecular Formula | (C14H21NO11)n |
| Molecular Weight | Variable, depends on crosslinking |
| Formulation | Injection, prefilled syringe with sterile needle |
| Strength | 60mg/3ml |
| Quantity | 1 syringe per box |
| Approval Number | HJ20233147 (China NMPA) |
| Product Code | 86981788000164 |
| Manufacturer | LG Chem, Ltd. (South Korea) |
| Barcode | Not officially recorded |
| Use | Laboratory research only |
| Storage Conditions | Store at 2°C–25°C; avoid direct sunlight and freezing |
Mechanism & Research Applications
BDDE-crosslinked hyaluronic acid resists enzymatic breakdown by forming ether bonds between polymer chains. This stabilization enhances viscosity, elasticity, and persistence, making it ideal for research involving:
Osteoarthritis models (OA)
Intra-articular lubrication studies
Cartilage regeneration and protection
Synovial fluid replacement research
Tissue engineering and 3D bioprinting support
BDDE-Cross Linked Sodium Hyaluronate Injection Side Effects (Observed in Preclinical Studies)
Although not intended for clinical use, preclinical and in vivo models may show:
Local swelling or inflammation at injection site
Temporary joint stiffness or mild pain
Rare hypersensitivity to crosslinkers (BDDE)
No systemic toxicity observed under standard dosing
Safety & Handling
| Category | Guidelines |
|---|---|
| Intended Use | Laboratory research only (in vitro/in vivo) |
| Not For Use In | Humans or animals in therapeutic/diagnostic contexts |
| Protective Gear | Gloves, lab coat, and safety goggles required during handling |
| Storage | Refrigerated (2–8°C) or below 25°C if labeled; avoid freezing |
| Disposal | Dispose according to local chemical and biological waste protocols |
Core Keywords
BDDE-cross linked sodium hyaluronate, hyaluronic acid injection 60mg 3ml, LG Chem BDDE HA, BDDE hyaluronic acid research, crosslinked sodium hyaluronate lab grade, osteoarthritis joint injection study, injectable HA for research, prefilled syringe sodium hyaluronate
Research Use Disclaimer
This product is intended solely for scientific research purposes. It is not approved for human therapeutic, diagnostic, or cosmetic use. Any misuse may result in regulatory or health risk. Users must follow institutional biosafety protocols and regional regulations.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |
Q1: Is this product approved for clinical or cosmetic use?
A1: No. It is approved for laboratory research only. Not for injection into humans or animals.
Q2: Does it come with a needle?
A2: Yes. Each unit includes a prefilled syringe and one sterile needle.
Q3: Is wholesale purchase available?
A3: Yes. Bulk and retail options are both supported.
Q4: What’s the crosslinker used?
A4: The crosslinking agent is BDDE (1,4-butanediol diglycidyl ether), widely accepted for stability.
Q5: Can I request a Certificate of Analysis (CoA)?
A5: Yes. CoA and SDS (Safety Data Sheet) are available upon request.













Reviews
There are no reviews yet.