No products in the cart.
Sale
Bendamustine Hydrochloride Injection CAS 3543-75-7 | Research-Grade Alkylating Agent
Original price was: $3.00.$2.00Current price is: $2.00.
High-purity Bendamustine Hydrochloride Injection designed for experimental research. Alkylating agent for in-vitro and ex-vivo studies of DNA damage, apoptosis pathways, and chemotherapeutic mechanism modeling. Strictly for laboratory use, non-clinical.
Description
Contents
hide
Product Description
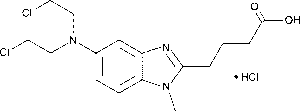
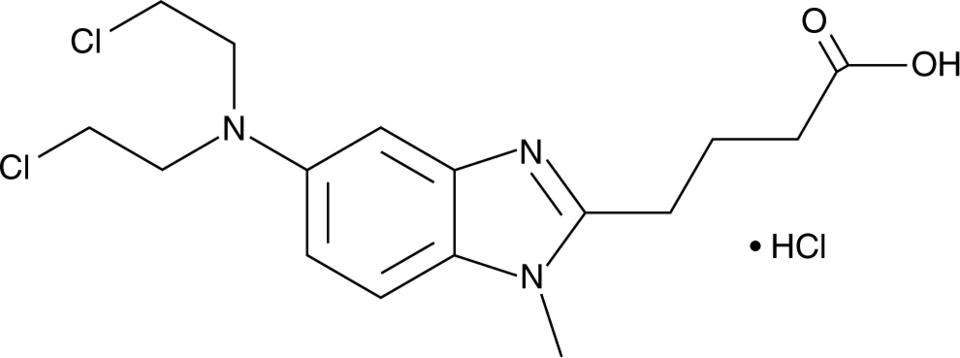
Bendamustine Hydrochloride Injection (CAS 3543-75-7) is a laboratory-grade alkylating agent with dual functional properties, acting both as a nitrogen mustard and a purine analog. In experimental models, it induces DNA cross-linking, replication stress, and apoptosis, providing researchers with a reproducible tool to study cellular responses to DNA damage in a controlled laboratory environment. Its high purity (>98%) and sterile injectable form ensure consistent activity in in-vitro and ex-vivo mechanistic studies.
In DNA damage response research, Bendamustine Hydrochloride facilitates mechanistic analysis of cell cycle checkpoints, repair pathway activation, and apoptotic signaling cascades. Researchers can examine γ-H2AX foci formation, p53-mediated responses, and caspase activation kinetics to delineate the cellular response to alkylating stress. This enables quantitative evaluation of DNA damage, repair efficiency, and programmed cell death in various laboratory models, including immortalized cell lines and primary cells.
Bendamustine Hydrochloride is also used to model chemotherapeutic mechanisms and resistance pathways in laboratory settings. It allows systematic investigation of alkylation-mediated cytotoxicity, synergistic interactions with other experimental compounds, and molecular determinants of cellular sensitivity. By providing a consistent and reproducible reagent, Bendamustine Hydrochloride supports studies into pathway cross-talk, compensatory survival signaling, and mechanistic pharmacology under strictly non-clinical research conditions.
Furthermore, its injectable form ensures homogeneity and eliminates variability associated with reconstitution from lyophilized powders. Researchers can reliably perform dose-response studies, time-course analyses, and pathway-specific readouts in controlled experimental setups. The compound serves as a reference standard for studying DNA damage, apoptosis induction, and alkylating agent mechanistic assays in laboratory-based cancer research, hematologic models, and mechanistic pharmacology studies.
Product Specifications
| Specification | Details |
|---|---|
| Compound Name | Bendamustine Hydrochloride |
| CAS Number | 3543-75-7 |
| Molecular Formula | C16H21Cl2N3O2 |
| Molecular Weight | 358.18 g/mol |
| Purity | ≥ 98% by HPLC |
| Form | Injectable solution |
| Appearance | Clear to slightly yellow sterile solution |
| Solubility | Water-soluble for laboratory use |
| Storage | 2–8°C, protect from light |
| Shelf Life | ≥ 12 months under recommended storage |
| Batch Documentation | Certificate of Analysis included |
| Laboratory Use | DNA damage and apoptosis mechanistic studies, in-vitro and ex-vivo research |
Mechanism of Action
Bendamustine Hydrochloride Injection (CAS 3543-75-7) functions as a bifunctional alkylating agent with distinctive mechanistic properties that are highly valuable in experimental research. Its nitrogen mustard moiety induces interstrand and intrastrand DNA cross-links by covalently binding nucleophilic sites within the DNA double helix, particularly at guanine residues. These cross-links disrupt DNA replication and transcription processes, generating replication stress and triggering the DNA damage response. In laboratory models, researchers can monitor these effects using γ-H2AX foci formation, comet assays, and other DNA-specific readouts to quantitatively assess DNA lesion formation.
The purine analog component of Bendamustine Hydrochloride further amplifies replication stress by interfering with nucleotide incorporation during DNA synthesis. This dual action results in the accumulation of single-strand and double-strand breaks, which activate canonical DNA damage signaling pathways such as ATM/ATR, Chk1/Chk2 phosphorylation, and p53 stabilization. Mechanistic studies in vitro and ex vivo can use these endpoints to analyze checkpoint activation, DNA repair engagement, and adaptive responses in experimental systems without clinical implications.
Apoptotic responses induced by Bendamustine Hydrochloride are primarily mediated through the intrinsic mitochondrial pathway. Laboratory assays demonstrate activation of pro-apoptotic Bcl-2 family proteins, mitochondrial outer membrane permeabilization, cytochrome c release, and sequential activation of caspases 9 and 3. Researchers can employ flow cytometry, immunofluorescence imaging, and Western blot analysis to dissect these processes, providing mechanistic insight into programmed cell death following DNA alkylation.
Beyond DNA cross-linking and apoptosis, Bendamustine Hydrochloride also influences cellular stress responses such as replication fork stalling and oxidative stress in laboratory settings. Mechanistic experiments can explore compensatory survival pathways, cross-talk between repair mechanisms, and chromatin remodeling events triggered by the compound. Integration with transcriptomic or proteomic analyses allows mapping of global cellular responses to DNA damage.
In summary, Bendamustine Hydrochloride serves as a reproducible experimental tool for studying DNA damage, replication stress, and apoptosis signaling. Its dual functional mechanism enables high-resolution mechanistic investigations in vitro and ex vivo, supporting robust laboratory research into alkylating agent biology, pathway regulation, and cellular stress responses.
Research Models
Bendamustine Hydrochloride Injection is widely employed in controlled laboratory models to investigate the mechanistic effects of DNA alkylation, replication stress, and apoptotic signaling. In vitro studies utilizing immortalized cell lines allow researchers to quantitatively assess DNA damage induction, checkpoint activation, and programmed cell death following exposure to the compound. These models provide a reproducible platform for mechanistic studies, enabling detailed mapping of cellular responses to alkylating agents without clinical implications.
Three-dimensional (3D) culture systems, such as spheroids and organoids, offer enhanced physiological relevance, allowing the evaluation of cellular interactions, microenvironmental effects, and tissue-level responses to DNA damage. Bendamustine Hydrochloride treatment in these models enables researchers to study replication stress propagation, apoptotic gradients, and heterogeneity in DNA repair capacity within a more tissue-like architecture. These insights are essential for understanding the dynamics of alkylation-induced cytotoxicity under experimentally controlled conditions.
Ex-vivo tissue slices and primary cell preparations provide additional mechanistic insight into organ- or tissue-specific responses. By applying Bendamustine Hydrochloride to these models, researchers can monitor DNA damage markers, apoptotic signaling, and repair pathway engagement within an intact extracellular matrix and native cellular microenvironment. Such experimental systems facilitate high-resolution studies of replication stress, cross-talk between cellular compartments, and mechanistic effects of dual-function alkylating activity under laboratory conditions.
Furthermore, Bendamustine Hydrochloride can be used in combination with experimental perturbations to explore pathway interactions, stress response kinetics, and compensatory mechanisms in diverse research models. Integrating readouts from flow cytometry, immunofluorescence, and high-content imaging with multi-omic analyses allows comprehensive mechanistic mapping. Collectively, these experimental models provide robust, reproducible platforms for dissecting DNA damage response, apoptosis induction, and mechanistic pharmacology, all within strictly non-clinical laboratory contexts.
Experimental Design Considerations
When designing experiments using Bendamustine Hydrochloride Injection (CAS 3543-75-7), careful planning is essential to achieve reproducible and mechanistic outcomes in laboratory studies. Researchers must first select appropriate experimental models, including 2D cell cultures, 3D spheroids, organoids, or ex-vivo tissue slices, to investigate DNA damage, replication stress, and apoptotic pathways. The choice of model directly impacts the observed cellular responses and the interpretability of mechanistic findings.
Controlled exposure to Bendamustine Hydrochloride is critical for consistent results. Researchers should establish experimental parameters, such as incubation times and relative concentrations, based solely on laboratory objectives, while ensuring safety and minimizing compound waste. Standardizing treatment conditions across replicates enhances reproducibility and allows precise comparison of DNA damage induction, apoptotic signaling, and pathway perturbation in mechanistic studies.
Appropriate selection of readouts is another key consideration when using Bendamustine Hydrochloride. Mechanistic assays commonly employed include γ-H2AX immunofluorescence for DNA damage, Western blotting for apoptosis markers, flow cytometry for cell cycle analysis, and high-content imaging to assess subcellular effects. Researchers should carefully align assay endpoints with specific study objectives to accurately capture the mechanistic impact of DNA cross-linking and replication stress.
Integration of Bendamustine Hydrochloride with complementary experimental techniques, such as multi-omic profiling or computational modeling, further enhances mechanistic insight. By combining transcriptomic, proteomic, or phospho-proteomic analyses with traditional DNA damage assays, researchers can map pathway dynamics, identify compensatory responses, and generate high-resolution datasets that inform broader experimental hypotheses.
Finally, strict laboratory safety and procedural controls are imperative when designing experiments with Bendamustine Hydrochloride. All manipulations should occur within certified fume hoods or biosafety cabinets, with appropriate personal protective equipment and decontamination procedures in place. Meticulous experimental planning, adherence to safety guidelines, and careful documentation of conditions ensure that Bendamustine Hydrochloride remains a reliable and reproducible tool for mechanistic research into DNA damage, apoptosis, and alkylating agent pathways.
Analytical Methods & Assays
Mechanistic studies typically employ γ-H2AX immunofluorescence, Western blotting for DNA damage and apoptotic markers, ELISA, flow cytometry, and imaging assays. Multi-omic approaches such as transcriptomics and proteomics can be integrated to examine global cellular responses to DNA damage. These methods provide high-resolution insights into mechanistic pathways while maintaining laboratory reproducibility and safety.
Integration with Multi-Omic & Computational Studies
Bendamustine Hydrochloride Injection (CAS 3543-75-7) provides a robust platform for integration with multi-omic and computational approaches in laboratory research. Its reproducible DNA-damaging and apoptotic effects allow researchers to generate high-quality mechanistic data suitable for transcriptomic, proteomic, and phospho-proteomic analyses. By applying Bendamustine Hydrochloride in controlled experimental models, investigators can systematically map pathway perturbations, quantify replication stress responses, and evaluate downstream signaling cascades in response to alkylating stress.
Transcriptomic profiling following Bendamustine Hydrochloride exposure enables identification of differentially expressed genes associated with DNA repair, checkpoint activation, and apoptotic regulation. Proteomic and phospho-proteomic analyses complement these studies by revealing dynamic post-translational modifications, protein interactions, and signaling network adaptations. Integration of these multi-omic datasets provides a comprehensive mechanistic view of cellular responses to Bendamustine Hydrochloride without implying human-use applicability.
Computational modeling further enhances the utility of Bendamustine Hydrochloride in experimental research. Systems biology approaches can simulate DNA damage response kinetics, apoptosis induction pathways, and replication stress propagation. Researchers can use these models to predict cellular outcomes, test mechanistic hypotheses, and design follow-up experiments with high precision. Coupling experimental readouts from Bendamustine Hydrochloride studies with computational analyses facilitates identification of regulatory nodes, feedback loops, and compensatory mechanisms in DNA damage and apoptosis pathways.
Overall, the integration of Bendamustine Hydrochloride with multi-omic and computational methodologies enables high-resolution mechanistic investigations. This approach provides detailed insights into the molecular regulation of DNA damage responses, apoptotic signaling, and cellular stress networks, supporting reproducible, non-clinical laboratory research with robust translational potential in mechanistic studies.
Applications
Bendamustine Hydrochloride is extensively used for:
Mechanistic studies of DNA cross-linking and apoptosis
Pathway mapping of DNA damage response and repair mechanisms
Evaluation of laboratory-based chemotherapeutic synergy or resistance
Multi-omic experiments to study stress response networks
Reference compound for benchmarking alkylating agents in mechanistic research
Laboratory Safety & Handling Guidelines
Handling Bendamustine Hydrochloride Injection (CAS 3543-75-7) requires strict adherence to laboratory safety protocols to ensure both researcher safety and compound integrity. As a bifunctional alkylating agent, Bendamustine Hydrochloride has potent DNA-damaging properties in experimental systems, and exposure must be strictly controlled. Researchers should always operate within designated laboratory areas equipped for handling cytotoxic or DNA-reactive compounds, ideally in certified chemical fume hoods or biosafety cabinets with appropriate airflow and filtration systems.
Personal protective equipment (PPE) is essential when working with Bendamustine Hydrochloride. Laboratory personnel should wear chemically resistant gloves, lab coats or gowns, and full eye protection, including safety goggles or face shields, to prevent accidental contact. In addition, respiratory protection should be considered in scenarios where aerosol generation is possible, such as during solution preparation or experimental manipulations that involve splashing or nebulization. Strict use of PPE minimizes risk of dermal or mucosal exposure and ensures reproducibility of experimental outcomes.
All handling procedures should incorporate measures to prevent cross-contamination of laboratory surfaces, instruments, and other reagents. Benchtops should be lined with absorbent laboratory paper or chemical-resistant mats, and all equipment should be decontaminated with appropriate DNA-destroying or cytotoxic-safe cleaning solutions after use. Pipettes, centrifuges, and other laboratory instruments in contact with Bendamustine Hydrochloride should be dedicated or thoroughly decontaminated to maintain experimental integrity and prevent unintentional exposure to other laboratory materials.
Storage and transportation within the laboratory environment must also be carefully managed. Bendamustine Hydrochloride Injection should be stored at 2–8°C, protected from light and moisture, in clearly labeled, leak-proof containers. Segregation from incompatible chemicals is necessary to avoid degradation or accidental reactions. When moving the compound between laboratory spaces, secondary containment trays or transport boxes should be used to contain potential spills and prevent accidental release.
In the event of accidental exposure or spill, laboratories must have clear emergency protocols. Skin or eye contact should be immediately rinsed with copious amounts of water, and exposed individuals should seek medical consultation in accordance with institutional safety guidelines, even though the compound is strictly for laboratory research. Spills should be contained using absorbent materials, and contaminated waste must be disposed of in accordance with institutional hazardous waste protocols. Special attention must be paid to the decontamination of surfaces, instruments, and any disposable materials that may have come into contact with the compound.
Researchers are strongly advised to maintain comprehensive laboratory records, including batch documentation, usage logs, and procedural details, to ensure both reproducibility of experimental studies and compliance with institutional safety standards. Training in cytotoxic and DNA-reactive compound handling is essential, and only personnel with demonstrated competency should manipulate Bendamustine Hydrochloride. Routine review of laboratory safety protocols and participation in refresher training helps ensure that safety measures remain current and effective.
Finally, all laboratory experiments with Bendamustine Hydrochloride should be designed to minimize the quantity of compound used while still achieving reliable mechanistic results. Implementing micro-scale assays, minimizing handling steps, and employing automated or semi-automated procedures where possible reduces exposure risk and promotes safer laboratory practices. By strictly following these guidelines, researchers can safely harness the mechanistic potential of Bendamustine Hydrochloride for in-vitro and ex-vivo studies while maintaining a controlled, compliant laboratory environment.
Side Effects
In laboratory research settings, Bendamustine Hydrochloride Injection (CAS 3543-75-7) induces well-characterized cellular and molecular effects that provide mechanistic insight into DNA damage and apoptosis pathways. Its bifunctional alkylating activity results in the formation of interstrand and intrastrand DNA cross-links, which subsequently disrupt replication and transcription processes in experimental models. These effects are not indicative of clinical toxicity but represent reproducible laboratory observations critical for mechanistic studies.
Exposure in in-vitro and ex-vivo models often leads to replication stress, checkpoint activation, and DNA damage accumulation. Researchers can detect γ-H2AX foci formation, replication fork stalling, and activation of DNA damage response proteins such as ATM, ATR, Chk1, and Chk2. These molecular events allow precise quantification of DNA lesions and cellular stress responses under controlled experimental conditions.
Apoptotic effects are a direct consequence of DNA damage and replication stress. Bendamustine Hydrochloride triggers mitochondrial outer membrane permeabilization, cytochrome c release, and caspase activation, leading to programmed cell death in laboratory models. These mechanistic outcomes are widely utilized to study intrinsic apoptotic signaling, Bcl-2 family protein regulation, and pathway cross-talk in cellular stress networks.
Additional experimental observations include transient cell cycle arrest, alterations in chromatin structure, and induction of oxidative stress markers. Researchers use these laboratory outcomes to investigate pathway interactions, compensatory responses, and the mechanistic effects of alkylating agents in DNA repair and apoptosis. It is important to note that these side effects are specific to experimental systems and do not reflect human or veterinary clinical responses.
Overall, the reproducible mechanistic effects of Bendamustine Hydrochloride in laboratory models make it a valuable tool for studying DNA damage, replication stress, and apoptosis pathways. Careful experimental design, controlled exposure, and appropriate readouts allow researchers to dissect cellular responses and molecular mechanisms with high resolution, supporting robust and reproducible scientific investigations in non-clinical settings.
Keywords
Bendamustine Hydrochloride, alkylating agent, research-grade, DNA damage, apoptosis pathways, mechanistic study, laboratory chemotherapeutic model, in-vitro DNA cross-linking, ex-vivo apoptosis analysis, DNA repair mechanistic research, pathway perturbation, laboratory pharmacology, replication stress, γ-H2AX foci, non-clinical research ,Cancer research
Shipping Guarantee
Bendamustine Hydrochloride Injection is shipped under strictly controlled laboratory conditions to ensure compound integrity. Secure packaging prevents contamination, protects against light exposure, and minimizes the risk of temperature fluctuations during transit. Each shipment is fully traceable, with batch documentation provided for experimental reproducibility. This ensures researchers receive the product in consistent condition, ready for laboratory mechanistic studies.
Trade Assurance
Factory-manufactured Bendamustine Hydrochloride is supplied with complete Certificates of Analysis (CoA) for each batch. Bulk and wholesale options are available to support high-throughput experimental research. Consistent purity and quality guarantee reproducibility of laboratory assays. Researchers can rely on the product for standardized mechanistic studies across multiple experiments and projects.
Payment Support
Multiple secure payment options are supported for laboratory and research institutions, including wire transfers, verified online payment platforms, and institutional accounts. Payment processing adheres to strict financial security protocols to ensure safe transactions. Flexible terms allow researchers to efficiently procure Bendamustine Hydrochloride for controlled experimental studies. This facilitates uninterrupted access for mechanistic and laboratory research applications.
Disclaimer
Bendamustine Hydrochloride Injection is strictly intended for laboratory and experimental research purposes. It is not for human or veterinary use, ingestion, or clinical administration. Researchers must follow all standard laboratory safety protocols, including PPE usage and controlled storage conditions. Adherence to institutional guidelines ensures safe handling and reproducible experimental outcomes.
References

Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
What are common experimental uses of Bendamustine Hydrochloride?
In research settings, Bendamustine Hydrochloride is primarily used to induce DNA cross-linking, replication stress, and apoptotic pathways in in‑vitro and ex‑vivo models. It enables mechanistic studies of DNA damage response, checkpoint signaling, and programmed cell death.
How does Bendamustine Hydrochloride induce DNA damage?
It functions as a bifunctional alkylating agent, forming both interstrand and intrastrand DNA cross-links via its nitrogen mustard moiety. This leads to replication stress, triggering DNA damage response pathways such as ATM/ATR and p53 signaling.
Does Bendamustine Hydrochloride trigger apoptosis in laboratory models?
Yes, DNA damage caused by Bendamustine often leads to activation of the intrinsic mitochondrial apoptotic pathway, including cytochrome c release and caspase‑9 / caspase‑3 activation.
Can Bendamustine Hydrochloride induce mitotic catastrophe?
In certain experimental models (e.g., cell lines resistant to other alkylators), Bendamustine can disrupt mitotic checkpoints, downregulate Aurora kinase A and PLK‑1, and cause mitotic catastrophe.
How is DNA repair affected by Bendamustine Hydrochloride in mechanistic studies?
Compared to some alkylating agents, Bendamustine-induced double-strand breaks are repaired more slowly, and it engages base-excision repair rather than classical alkyltransferase mechanisms.
What experimental readouts are typically used with Bendamustine Hydrochloride?
Researchers often monitor γ‑H2AX foci for DNA damage, checkpoint kinase activation (e.g., Chk1/Chk2), p53 stabilization, flow cytometry for apoptosis, and caspase activation by Western blot.
Can Bendamustine Hydrochloride be used in multi‑omic studies?
Yes, it is compatible with transcriptomic, proteomic, and phospho‑proteomic analyses, enabling mapping of gene expression, signaling cascades, and post‑translational modifications in response to DNA damage.
Is Bendamustine Hydrochloride stable in storage for experimental use?
For laboratory-grade formulations, it should be stored under recommended conditions (e.g., 2–8°C, protected from light) to maintain chemical integrity and reproducible activity.
What safety precautions should be taken when handling Bendamustine in the lab?
Use personal protective equipment (gloves, lab coat, eye protection), handle within a chemical fume hood or biosafety cabinet, and decontaminate surfaces and instruments after use to avoid cross-contamination.
Are there specific cell models that respond well to Bendamustine Hydrochloride?
Yes — both immortalized hematological cell lines and primary cells are frequently used to study its effects, including DNA damage, checkpoint activation, and apoptosis.
Can Bendamustine Hydrochloride be used to benchmark other DNA‑targeting agents?
Absolutely. Its reproducible mechanistic activity makes it a valuable reference compound to compare with newer alkylating agents or DNA-damaging reagents in experimental assays.
Does Bendamustine Hydrochloride cause oxidative stress in lab models?
Yes, in some mechanistic studies it induces oxidative stress and replication fork stalling, which can be quantified via ROS assays, replication dynamics, and stress-signaling readouts.
Is the mitotic arrest observed after Bendamustine treatment reversible in experimental models?
That depends on the model: in some in‑vitro systems, cells may recover or die by mitotic catastrophe depending on checkpoint activation and repair capacity; mechanistic assays help clarify these outcomes.














Reviews
There are no reviews yet.