No products in the cart.
Sale
Bleomycin Hydrochloride | High Purity 98.92% | GMP Supplier for Research
Original price was: $26.00.$22.00Current price is: $22.00.
Bleomycin hydrochloride is a glycopeptide antibiotic widely used in cancer research for its ability to induce DNA strand breaks and inhibit cell proliferation. It serves as a model compound in studies of DNA damage, repair mechanisms, and oxidative stress pathways.
Description
Product Description
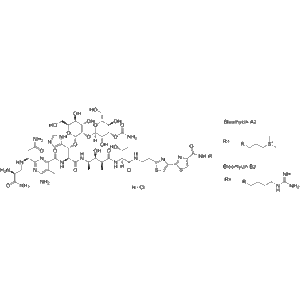
Bleomycin hydrochloride is a complex glycopeptide antibiotic derived from Streptomyces verticillus. It belongs to the bleomycin family of cytotoxic compounds, known for their DNA-cleaving properties. In biochemical and molecular biology research, bleomycin hydrochloride is a powerful tool for inducing double-stranded DNA breaks, enabling the investigation of cellular responses to DNA damage, oxidative stress, and apoptosis.
Structurally, bleomycin hydrochloride is composed of a metal-binding domain, a bithiazole region that intercalates into DNA, and a carbohydrate moiety that enhances solubility. The compound exerts its primary effect by chelating metal ions, particularly Fe(II), and reacting with oxygen to form reactive oxygen species (ROS). These radicals attack DNA, resulting in single- and double-strand breaks that trigger cellular repair mechanisms.
In cancer-related research, bleomycin hydrochloride serves as a prototype for DNA-damaging agents. It has been utilized to model genotoxic stress in various tumor cell lines, helping researchers understand pathways such as p53 activation, chromatin remodeling, and cell cycle arrest. Its selective toxicity toward rapidly proliferating cells makes it valuable for exploring therapeutic strategies and drug resistance mechanisms.
Beyond oncology, bleomycin hydrochloride plays an essential role in studying oxidative damage and cellular senescence. It mimics the oxidative DNA injury that occurs during aging and exposure to environmental toxins. This property allows researchers to analyze how cells detect and repair oxidative lesions and how chronic DNA stress contributes to genomic instability.
Bleomycin hydrochloride is also widely employed in gene therapy vector testing, radiation biology, and fibrosis modeling. In fibroblast systems, it induces controlled tissue injury, allowing for the evaluation of fibrogenic signaling and extracellular matrix deposition. Such models are critical for understanding pulmonary and dermal fibrosis mechanisms.
As a standard reagent in cell biology, bleomycin hydrochloride enables researchers to calibrate DNA repair assays, study the kinetics of double-strand break repair, and evaluate the efficacy of novel DNA-protective agents. The compound’s reliability and reproducibility make it a preferred reagent for controlled DNA damage induction in both in vitro and in vivo models.
Its utility extends to molecular imaging and biophysical analysis, where bleomycin analogs have been conjugated with fluorescent or radiolabeled tags to visualize DNA interaction. These derivatives support the study of DNA–protein complexes, chromatin dynamics, and metal–oxygen chemistry in biological systems.
Overall, bleomycin hydrochloride remains a cornerstone compound in experimental oncology and molecular toxicology. Its precise mechanism of DNA cleavage, coupled with its versatility across multiple disciplines, ensures its continued relevance as a research-grade reagent.
Product Specifications
| Property | Description |
|---|---|
| Product Name | Bleomycin hydrochloride |
| CAS Number | 67763-87-5 |
| Synonyms | Bleomycin sulfate; Bleomycin A2 hydrochloride |
| Molecular Formula | C₅₅H₈₄N₁₇O₂₁S₃·HCl |
| Molecular Weight | 1415.4 g/mol |
| Appearance | White to off-white lyophilized powder |
| Purity | ≥99% |
| Solubility | Soluble in water and methanol |
| Storage | Store at -20°C, protected from light and moisture |
| Stability | Stable under recommended storage conditions |
| Category | Glycopeptide antibiotic |
| Applications | DNA damage induction, oxidative stress studies, fibrosis modeling, cancer research |
| Intended Use | For laboratory research use only |
Mechanism of Action
Bleomycin hydrochloride exerts its biological activity through a well-characterized metal-mediated oxidative mechanism. The compound binds divalent metal ions—commonly Fe(II)—forming a bleomycin–iron complex capable of reacting with molecular oxygen. This reaction generates an activated bleomycin–Fe(III)–OOH species, which functions as a pseudo-hydroxyl radical source. The resulting oxidative attack on DNA leads to site-specific cleavage of phosphodiester bonds, causing both single- and double-strand breaks.
The DNA-binding domain of bleomycin hydrochloride, particularly the bithiazole moiety, intercalates between base pairs. This intercalation positions the metal-reactive domain near the deoxyribose backbone, allowing for efficient hydrogen abstraction and oxidative cleavage. Studies have shown that guanine and cytosine-rich regions are preferential targets, leading to preferential strand scission at these sequences.
Bleomycin-induced DNA damage activates several downstream cellular responses. In mammalian systems, it triggers phosphorylation of histone H2AX, recruitment of DNA repair proteins (such as ATM, ATR, and BRCA1), and initiation of apoptosis in cases of irreparable damage. These pathways are essential for understanding how cells maintain genomic integrity under genotoxic stress.
The mechanism also explains its antifibrotic and oxidative stress model applications. In fibroblast cultures, bleomycin exposure activates transforming growth factor-beta (TGF-β) signaling, leading to collagen synthesis and extracellular matrix accumulation. This property makes it a reproducible agent for modeling pulmonary fibrosis and testing antifibrotic interventions.
Furthermore, the ability of bleomycin hydrochloride to simulate oxidative DNA injury provides a valuable system for antioxidant evaluation. By quantifying ROS levels, lipid peroxidation, and DNA fragmentation in treated cells, researchers can assess the efficacy of novel ROS-scavenging compounds.
Bleomycin’s selectivity is attributed to cellular uptake mechanisms mediated by specific transporters such as hCT2 (human copper transporter). The concentration-dependent cytotoxic profile also provides insight into pharmacodynamic relationships between oxidative damage and cell survival signaling.
In conclusion, bleomycin hydrochloride’s dual capacity to act as a DNA-cleaving and oxidative stress-inducing agent makes it indispensable in mechanistic studies of DNA metabolism, cell death, and repair fidelity.
Side Effects
In research settings, bleomycin hydrochloride exhibits a range of cellular and molecular effects that correspond to its DNA-damaging activity. Cytotoxicity arises primarily from the accumulation of double-strand DNA breaks and activation of apoptosis pathways. Depending on concentration and exposure duration, cells may undergo senescence, necrosis, or apoptotic death.
In mammalian models, bleomycin has been associated with dose-dependent oxidative stress, mitochondrial dysfunction, and activation of inflammatory mediators. Prolonged exposure in fibroblast or epithelial cultures can lead to overexpression of fibrogenic cytokines and deposition of extracellular matrix proteins, forming the basis for fibrosis research models.
Although bleomycin hydrochloride is used exclusively for research purposes, standard laboratory safety protocols are essential. Handling should be performed in a controlled environment, with appropriate personal protective equipment and waste disposal procedures to minimize accidental exposure.
Keywords
Bleomycin Hydrochloride, CAS 67763-87-5, DNA synthesis inhibitor, DNA damaging agent, antitumor antibiotic research, apoptosis research compound, DNA repair inhibitor, oncology laboratory chemical, GMP Bleomycin Hydrochloride supplier, wholesale research chemicals
Shipping Guarantee
All shipments are handled using validated cold-chain logistics to preserve peptide integrity. Each package is sealed in moisture-proof containers with secondary protective wrapping and continuous temperature monitoring. Products are shipped via express international couriers with full tracking and insurance coverage.
Trade Assurance
We ensure product authenticity, verified ≥99% purity, and compliance with analytical standards (HPLC, MS, and NMR). Each batch is supplied with a Certificate of Analysis (CoA). Our trade assurance policy guarantees replacement or refund for any deviation from listed specifications.
Payment Support
We provide flexible and secure global payment options to support international research transactions. Accepted payment methods include PayPal, major credit cards (Visa, MasterCard, American Express), telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are protected by industry-standard encryption and verified payment gateways to ensure confidentiality and fund security.
Disclaimer
For laboratory research use only. Not intended for human or animal administration. No therapeutic, diagnostic, or clinical applications are implied. Researchers should handle this compound under appropriate biosafety conditions.
Additional information
| Weight | 0.6 kg |
|---|---|
| Dimensions | 82 × 64 × 82 cm |
1 review for Bleomycin Hydrochloride | High Purity 98.92% | GMP Supplier for Research
What is Bleomycin Hydrochloride?
Bleomycin Hydrochloride is an antitumor antibiotic and DNA damaging agent that inhibits DNA synthesis. It is used in laboratory research for studying cancer biology and DNA repair pathways.
What is the CAS number of Bleomycin Hydrochloride?
The CAS number for Bleomycin Hydrochloride is 67763-87-5.
What purity is available for Bleomycin Hydrochloride?
This product is offered at a high purity of 98.92%, ensuring accuracy in laboratory experiments.
What are the primary research uses of Bleomycin Hydrochloride?
It is used in oncology research, DNA repair studies, apoptosis induction experiments, and models of drug resistance.
How does Bleomycin Hydrochloride work?
It binds DNA in the presence of iron and oxygen, causing DNA strand breaks and cell cycle arrest.
Is Bleomycin Hydrochloride GMP certified?
Yes, this compound is manufactured under GMP-certified conditions, ensuring reliability and reproducibility for laboratory studies.
Can Bleomycin Hydrochloride be used in humans or animals?
No. It is strictly intended for laboratory research use only and is not suitable for human or veterinary applications.
What form is Bleomycin Hydrochloride supplied in?
It is typically supplied as a white to off-white powder, suitable for dissolution under laboratory protocols.
Why is Bleomycin Hydrochloride important in research?
It is a reliable model compound for DNA damage and apoptosis induction, making it essential in cancer research and DNA repair studies.
Does Bleomycin Hydrochloride support wholesale orders?
Yes. We support both wholesale and retail supply to academic institutions, research laboratories, and biotech companies.













daubie –
The logistics took too long, I waited for 12 days