No products in the cart.
Bupivacaine Hydrochloride 37.5?mg ×?5 Vials Wholesale price comparison
$2.00
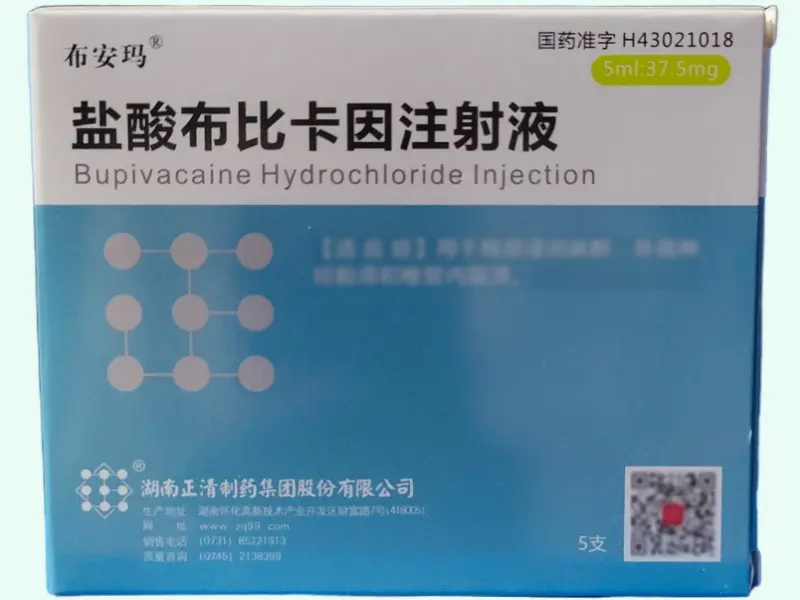
Bupivacaine hydrochloride is a long-acting amide-type local anesthetic widely used in preclinical studies for nerve block, infiltration, epidural, and spinal anesthesia. Each box contains five 5?mL vials (37.5?mg per vial). Manufactured by Hunan Zhengqing Pharmaceutical Group Co., Ltd., approved under NMPA H43021018, product code 86904152004254.
?? For laboratory research use only.?Please consult the staff for other purposes?
Description
Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Bupivacaine Hydrochloride Injection (Bu’anma®) |
| Generic Name | Bupivacaine Hydrochloride |
| CAS Number | 38396?39?3 / 73360?54?0 accessdata.fda.gov+9store.usp.org+9shharvest.com+9pubchem.ncbi.nlm.nih.govstore.usp.org+2en.wikipedia.org+2zh.wikipedia.org+2 |
| Molecular Formula | C??H??N?O·HCl·H?O |
| Molecular Weight | ~342.9?Da |
| Dosage Form | Solution for injection (sterile) |
| Strength | 37.5?mg per 5?mL vial |
| Pack Size | 5 vials per box |
| Approval Number | H43021018 (NMPA, China) |
| Product Code | 86904152004254 |
| Manufacturer | Hunan Zhengqing Pharmaceutical Group Co., Ltd. |
| Barcode | 6922670003720 (verify regionally) |
| Storage | Store at 2–25?°C, protect from light |
| Intended Use | Laboratory research use only |
Mechanism of Action
Bupivacaine blocks voltage-gated sodium channels in neuronal membranes, preventing depolarization and transmission of pain signals. Onset occurs within 5–10 minutes with a duration lasting 2–8 hours—significantly longer than lidocaine.
Research Applications & Pharmacology
Nerve blocks & regional anesthesia: Peripheral, epidural, spinal, or infiltration anesthesia in rodent and large-animal studies .
Analgesic pharmacodynamics: Onset typically within 5–10 minutes, peak at ~15–20 minutes, lasting 3–6 hours or longer .
Pharmacokinetics: ~95% protein binding, hepatic metabolism, renal excretion; half-life ~3.1?hr in adults .
Safety Profile & Handling
Adverse effects:
Cardiovascular toxicity (bradycardia, hypotension, arrest) in overdose or IV administration .
CNS effects: dizziness, headache, muscle twitching, risk of seizures .
Handling precautions:
Use proper PPE and biosafety procedures.
Monitor cardiovascular and respiratory status in animal models.
Avoid rapid IV injection—aspirate before administration to avoid intravascular delivery.
Dispose of used vials as pharmaceutical hazardous waste.
?? Research?Use Disclaimer
This product is intended exclusively for laboratory research. It is not approved for clinical, diagnostic, therapeutic, or veterinary use. Misuse may pose significant health risks and compromise research integrity.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |










Reviews
There are no reviews yet.