No products in the cart.
Sale
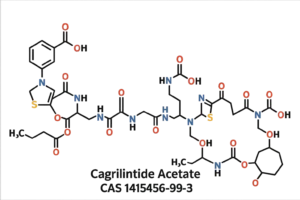
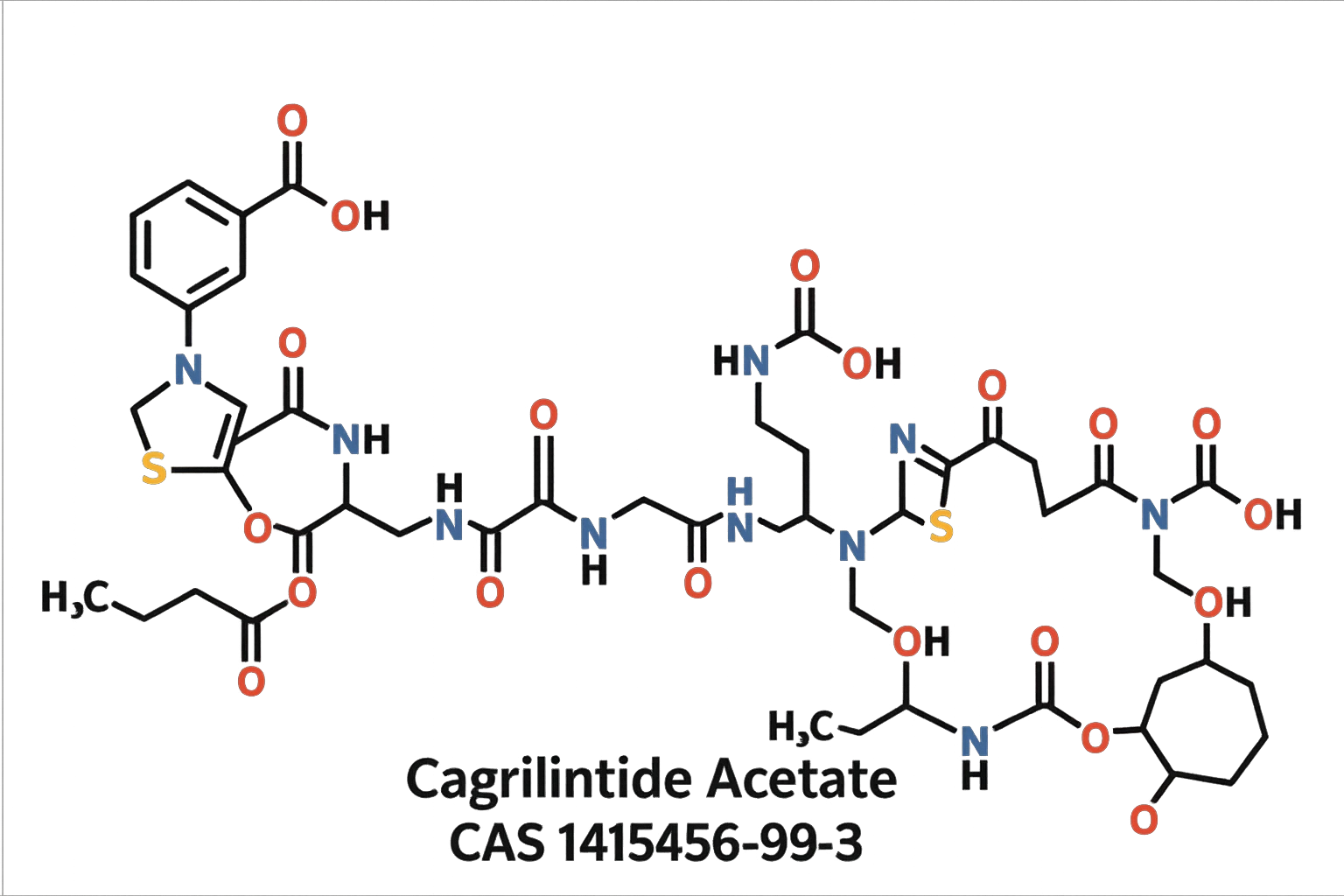
Cagrilintide Acetate CAS 1415456-99-3 | Freeze-Dried Powder High-Purity Wholesale
Original price was: $32.00.$28.00Current price is: $28.00.
Cagrilintide Acetate (CAS 1415456-99-3) is a high-purity freeze-dried peptide supplied for laboratory research. It is ideal for studies of glucagon and amylin receptor modulation, metabolic regulation, and peptide-based experimental workflows.(For research use only. Not for human or veterinary use.)
Description
Contents
hide
Product Description
Cagrilintide Acetate (CAS 1415456-99-3) is an advanced, next-generation long-acting amylin analogue developed to modulate appetite, reduce caloric intake, and improve metabolic efficiency in controlled research environments. Supplied as a high-purity freeze-dried powder, this molecule is engineered for optimal stability, reproducibility, and versatility across pharmacology, endocrinology, and metabolic disease research. Its extended half-life and enhanced receptor affinity make it an exceptional tool for studies involving body-weight regulation, satiety signaling, gastrointestinal motility, and energy-balance pathways.
As a structurally optimized amylin mimetic, Cagrilintide Acetate demonstrates robust activity at amylin and calcitonin receptors, enabling researchers to investigate multi-pathway mechanisms underlying obesity, hyperphagia, and related metabolic dysfunction. The compound’s long-acting pharmacokinetic profile distinguishes it from traditional amylin analogues, offering more stable plasma levels and reduced dosing frequency during experimental timelines. These characteristics make the molecule especially valuable in chronic-administration models and combination-therapy studies—for example, when deployed alongside incretin-pathway components such as GLP-1 or GIP agonists.
Manufactured under GMP-aligned factory conditions, the freeze-dried (lyophilized) form ensures excellent integrity, minimal degradation, and enhanced solubility upon reconstitution. Each batch of Cagrilintide Acetate undergoes strict analytical validation using HPLC, MS, and microbiological testing, guaranteeing consistent purity, potency, and contamination-free performance for high-sensitivity research applications. Bulk and wholesale orders can be customized for purity grades, vial sizes, packaging specifications, and labeling to meet laboratory, institutional, or commercial R&D needs.
Cagrilintide Acetate is widely applied in metabolic-disease research frameworks, including studies involving weight-loss mechanisms, nutrient-absorption kinetics, delayed gastric emptying, food-intake regulation, and neural-endocrine feedback loops. Because of its similarity to endogenous amylin activity, the molecule is also suited for work involving pancreatic hormone pathways, glucose-homeostasis modeling, and multi-omic data integration for mechanistic mapping.
Given the rising interest in amylin-pathway agents for obesity pharmacology, Cagrilintide Acetate serves as a key research standard for evaluating novel therapeutic combinations, dose-response relationships, and mechanistic endpoints. Researchers utilize this compound to explore synergy relationships between satiety-inducing peptides, uncover appetite-modulating neurocircuitry, and characterize metabolic biomarkers across transcriptomic, proteomic, and systems-biology datasets.
Every unit of Cagrilintide Acetate freeze-dried powder is securely packaged with temperature-controlled protection, tamper-evident seals, and complete batch documentation, enabling safe international transport and dependable long-term storage. This product is strictly intended for laboratory research use and is not approved for human administration, clinical treatment, or diagnostic procedures.

Product Specifications
| Specification | Details | Notes |
|---|---|---|
| Product Name | Cagrilintide Acetate | Full chemical name for cataloging. |
| CAS Number | 1415456-99-3 | Unique chemical identifier. |
| Molecular Formula | C<sub>167</sub>H<sub>252</sub>N<sub>44</sub>O<sub>52</sub>S<sub>1</sub> | Exact peptide composition. |
| Molecular Weight | 3830.9 Da | For solution preparation and analysis. |
| Purity | ≥98% (HPLC) | Verified by HPLC and mass spectrometry. |
| Appearance | White to off-white freeze-dried powder | Lyophilized form improves stability. |
| Solubility | Sterile water, PBS, DMSO | Avoid strong acids or bases; prepare fresh aliquots. |
| Reconstitution Concentration | 1–10 mg/mL typical | Adjust for in vitro assays; mix gently. |
| Storage Conditions | -20°C or lower; protect from light/moisture | Use airtight vials and desiccants. |
| Stability | ≥12 months | Verified under controlled lab conditions. |
| Form | Lyophilized powder | Enhances shelf-life and research consistency. |
| Applications | Biochemical assays, receptor binding, molecular mechanism studies | For laboratory research only. |
| Bulk/Wholesale Availability | Gram to kilogram quantities | Factory-direct supply; customizable packaging. |
| Quality Assurance | COA provided | Confirms identity, purity, analytical verification. |
| Analytical Methods | HPLC, Mass Spectrometry, Peptide Sequencing | Supports reproducibility and verification. |
| Safety Classification | Research-use only | Not for human or veterinary use. |
| Structure Representation | [Insert structure image here] | Optional visual for mechanistic reference. |
Stability Guidelines
Freeze-dried Cagrilintide Acetate is stable for over 12 months at -20°C when protected from light and moisture.
Reconstituted peptide is stable up to 48 hours at 4°C; avoid extended room temperature exposure.
Aliquoting is recommended to minimize repeated thaw cycles and maintain experimental consistency.
Store in amber vials or shielded containers to prevent light-induced degradation.
Solubility and Concentration Recommendations
| Solvent | Recommended Concentration | Notes |
|---|---|---|
| Sterile Water | 1–10 mg/mL | Prepare fresh for each experiment. |
| PBS (pH 7.2–7.4) | 1–5 mg/mL | Compatible with receptor binding and molecular assays. |
| DMSO | ≤10 mg/mL | Suitable for molecular studies; dilute appropriately before use. |
Mechanism of Action
Cagrilintide Acetate is a long-acting amylin analogue designed to interact with calcitonin receptor (CTR) complexes that are functionally paired with receptor activity–modifying proteins (RAMPs). These receptor assemblies behave as amylin-responsive signaling units that participate in satiety regulation, metabolic balance, and nutrient-related neural modulation. Through stable interaction with these receptor complexes, the molecule enables prolonged receptor engagement and extended signaling periods that researchers frequently explore in metabolic and neuroendocrine studies.
At the cellular signaling level, Cagrilintide Acetate is believed to influence second-messenger cascades, including cAMP-dependent pathways, which may affect downstream transcriptional regulators associated with energy sensing and appetite-modulating networks. Researchers also examine how Cagrilintide-related signaling may alter communication between hypothalamic nuclei, particularly those involved in satiety perception, sensory integration, and metabolic adaptation.
In metabolic research frameworks, Cagrilintide Acetate is commonly evaluated for its ability to modulate gastric motility-related signaling patterns, contributing to extended post-nutrient satisfaction windows and altered neurochemical feedback cycles. The compound’s structural stability and long receptor-residence time make it an attractive candidate for experiments focused on sustained anorectic-like activity, receptor occupancy kinetics, and neural integration within satiety pathways.
To support computational studies and receptor-binding modeling, Cagrilintide Acetate is also utilized in in silico simulations that investigate ligand–receptor docking geometry, energy-minimization conformations, and interaction maps across CTR/RAMP interfaces. These simulations help researchers predict potential signaling outputs, receptor preferences, and allosteric interaction patterns that may not be easily observable through wet-lab techniques alone.
Applications
Cagrilintide Acetate is a versatile research peptide with applications spanning metabolic studies, obesity research, and translational pharmacology. Its dual amylin and GLP-1 receptor agonist activity makes it highly valuable for preclinical investigations across multiple domains.
1. Obesity and Weight Management Research
Investigates energy intake regulation through satiety signaling pathways.
Evaluates body weight reduction in diet-induced obesity (DIO) rodent models.
Supports studies on adiposity and fat mass reduction mechanisms.
Enables combination therapy research with other GLP-1 analogs or metabolic modulators.
2. Glycemic Control and Diabetes Studies
Explores glucose-dependent insulin secretion and pancreatic beta-cell function.
Examines glucagon suppression in hyperglycemic models.
Facilitates mechanistic studies on type 2 diabetes interventions.
Can be used to assess long-term glycemic stability in preclinical trials.
3. Appetite and Satiety Mechanism Studies
Modulates central nervous system pathways involved in feeding behavior.
Supports research into amylin receptor-mediated satiety signaling.
Evaluates behavioral and neurological responses to peptide administration.
4. Pharmacokinetics and Pharmacodynamics Research
Suitable for in vivo pharmacokinetic profiling in rodents and non-human primates.
Enables dose-response analysis for dual receptor agonists.
Supports comparative studies with other GLP-1 or amylin analogs.
5. Preclinical Combination Therapy Research
Facilitates evaluation of synergistic effects with incretin mimetics.
Supports multi-target metabolic intervention studies, including anti-obesity and anti-diabetic strategies.
Enables investigation of peptide co-agonists in translational medicine.
6. Cellular and Molecular Mechanistic Studies
Used in in vitro receptor binding assays for GLP-1 and amylin receptors.
Supports studies on signal transduction pathways, including cAMP, PKA, and calcium signaling.
Useful for gene expression analysis in metabolic and appetite-regulating cells.
7. Translational and Comparative Research
Applicable in non-human primate studies for translational efficacy data.
Helps bridge preclinical findings with potential human therapeutic development.
Enables cross-species pharmacology evaluation to optimize dosing strategies.
Summary:
Cagrilintide Acetate serves as a robust tool for comprehensive metabolic research, including obesity, diabetes, appetite regulation, pharmacokinetics, and combination therapy studies. Its versatility makes it suitable for both mechanistic investigations and translational research programs, supporting the development of novel metabolic therapeutics.

1. Obesity, Appetite Regulation & Body-Weight Control Research
Cagrilintide Acetate is extensively utilized in studies evaluating:
Suppression of food intake and alteration of feeding patterns
Enhancement of satiety signaling and long-acting appetite control
Interactions with NPY/AgRP, POMC, CART, and melanocortin pathways
Weight-loss mechanisms in high-fat diet animal models
Comparison with GLP-1 analogs, amylin analogs, and poly-agonist peptides
This makes it a central tool compound for developing next-generation anti-obesity therapeutics.
2. Glucose Metabolism & Insulin Sensitivity Research
Researchers employ Cagrilintide in models exploring:
Glucose tolerance and postprandial glucose control
Pancreatic β-cell response and insulin secretion modulation
Peripheral insulin sensitivity and hepatic glucose output
Multi-agonist synergy when combined with GLP-1 agonists such as semaglutide
This supports preclinical evaluation of dual glucose–weight management drug platforms.
3. Incretin & Glucagon System Combination Research
Cagrilintide is often used to study coordinated metabolic signaling between:
GLP-1 receptor pathways
Glucagon receptor–mediated lipid and energy expenditure
Amylin-based satiety circuits
Multi-hormonal synergy in metabolic disease models
Its multi-receptor activity makes it valuable for poly-agonist drug design frameworks.
4. Gastric Emptying, GI Motility & Satiety Hormone Studies
Cagrilintide supports research into:
Delayed gastric emptying and nutrient absorption kinetics
GI hormone regulation, including PYY, GLP-1, and CCK
Gut–brain satiety signaling
Feeding behavior modification through slowed gastric transit
These mechanisms are essential for understanding its prolonged fullness and reduced intake effects.
5. Long-Acting Peptide Therapeutics Design & Structural Optimization
Because Cagrilintide incorporates modifications that dramatically extend half-life, it is frequently used in:
Stability and half-life extension studies
Structure–activity relationship (SAR) analysis
Tissue distribution, binding kinetics, and clearance profiling
Evaluation of lipidation, amino-acid substitution, and peptide engineering strategies
This makes it a benchmark molecule for long-acting peptide engineering.
6. Metabolic Syndrome (MetS) & Lipid Regulation Models
Cagrilintide contributes to studies involving:
Dyslipidemia and fatty-acid oxidation regulation
Hepatic lipid accumulation and steatosis reduction
Weight-related inflammatory markers and cytokines
Multi-parameter MetS studies combining glycemic and lipid endpoints
This enhances its relevance across broad metabolic research domains.
7. Combination Therapy Research
Cagrilintide is a key tool for evaluating synergy with:
GLP-1 R agonists (semaglutide, liraglutide)
Dual GIP/GLP-1 agonists (tirzepatide)
Amylin analogs (pramlintide)
Glucagon agonists
Lipid metabolism modulators
Its consistent synergistic effects make it ideal for combination regimen optimization.
8. Behavioral Research & Feeding-Motivation Studies
Cagrilintide is used extensively in behavioral models, including:
Food-seeking behavior analysis
Fat vs sugar preference shifts
Satiety duration modeling
Reward-pathway influence on feeding behavior
These studies help evaluate central appetite-regulation activity in detail.
Research Models
Cagrilintide Acetate is suitable for multiple research model categories, particularly those focused on metabolic regulation, peptide-target interactions, and receptor signaling. In cell-based assays, it enables detailed exploration of receptor activation, downstream signaling cascades, second-messenger fluctuations, and peptide–cell surface interactions. These models can include engineered receptor-expressing cells, metabolic profiling systems, and peptide-sensing reporter lines.
In biochemical and biophysical models, the compound is widely used for receptor-ligand affinity assays, thermodynamic binding studies, and conformational analysis using techniques such as CD spectroscopy, SPR, ITC, and NMR. These platforms help researchers evaluate structural transitions, activation kinetics, and ligand–protein complex stability.
Computational models also incorporate Cagrilintide Acetate for machine-learning–assisted peptide modeling, structural prediction, interaction probability mapping, and large-scale simulations exploring signaling dynamics. Systems biology frameworks apply the compound to study pathway correlations, gene-regulatory networks, and metabolic flux distributions.
Additionally, researchers rely on the compound in multi-omic integrative models, which can include metabolomic maps, phosphoproteomic datasets, peptide activity matrices, and advanced pathway reconstruction. These models give a comprehensive view of how peptide signals propagate through intricate biological networks.
Experimental Design Considerations
When designing experiments involving Cagrilintide Acetate, researchers should prioritize consistent peptide handling methods, clear experimental variable control, and adequate replication. Its freeze-dried form allows flexible reconstitution, but it is essential to ensure complete dissolution, typically through gentle agitation rather than mechanical stress. Buffer choice should be compatible with peptide stability, avoiding extremes in pH or high ionic strength conditions that may alter structural integrity.
Batch consistency should be maintained across long-term studies, using identical lots when possible to minimize analytical variability. Researchers may validate peptide integrity using HPLC or MS prior to critical assays to confirm purity and stability. For signal-detection studies, calibration curves, appropriate controls, and exposure gradients are recommended for reliable interpretation.
Temperature-sensitive experiments should use controlled environments to prevent unintended peptide degradation or conformational shifts. Multi-platform research—such as combining biochemical assays with transcriptomic or computational analyses—requires standardized sample preparation workflows to ensure cross-experiment comparability.
Documentation of reagent handling, aliquoting procedures, and reconstitution records supports reproducibility for long-running research series and collaborative studies.
Laboratory Safety & Handling Guidelines
Cagrilintide Acetate (CAS 1415456-99-3) is a high-purity research peptide that must be handled with care to ensure both laboratory safety and product integrity. All handling of Cagrilintide Acetate should occur in a well-ventilated laboratory equipped with emergency showers and eyewash stations. Personnel must wear personal protective equipment (PPE) including lab coats, gloves, and safety goggles at all times to prevent direct skin or eye contact.
When working with lyophilized Cagrilintide Acetate powder, use biosafety cabinets or containment systems to avoid inhalation and cross-contamination. Reconstitution should be performed with sterile water or appropriate buffers under aseptic conditions, minimizing vigorous shaking to preserve peptide structure. To maintain stability, aliquot and store the peptide at -20°C, protected from moisture and light, avoiding repeated freeze-thaw cycles. Proper labeling of all vials with product name, CAS number, concentration, and hazard information is essential.
Waste management of Cagrilintide Acetate requires strict adherence to institutional chemical disposal protocols. Contaminated gloves, vials, and pipette tips should be placed in designated hazardous waste containers. Spill management involves containment, careful cleanup using absorbent materials, and surface decontamination with 70% ethanol or appropriate disinfectants. In the event of skin or eye exposure, rinse thoroughly with water and seek medical attention.
All laboratory personnel must be trained in safe handling, storage, and emergency procedures specific to Cagrilintide Acetate. Maintaining logs for storage conditions, usage, and waste disposal ensures traceability and compliance. By following these comprehensive laboratory safety and handling guidelines, researchers can effectively utilize Cagrilintide Acetate in preclinical studies while minimizing risk to personnel and preserving peptide integrity.
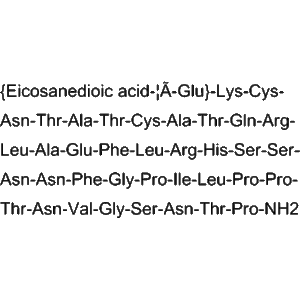
Integration with Multi-Omic & Computational Studies
Cagrilintide Acetate supports broad integration across transcriptomic, proteomic, metabolomic, and computational research platforms. In transcriptomics, the peptide is used for mapping changes in gene expression profiles tied to receptor engagement and metabolic signaling. High-throughput sequencing workflows can identify pathway modulation patterns and reveal regulatory network interactions.
Proteomics research leverages the compound to observe modifications in signaling proteins, phosphorylation cascades, and receptor-associated complexes. Mass spectrometry–based workflows are commonly used to quantify downstream biomarkers and peptide-responsive protein groups.
In metabolomics, researchers can explore shifts in metabolic intermediates, energy-balance markers, and nutrient-responsive pathways. These datasets can be correlated with proteomic and transcriptomic findings to build holistic metabolic maps.
Computational analyses—including structural modeling, peptide docking, pathway reconstruction, and machine-learning prediction—allow researchers to examine potential interaction landscapes, receptor conformational states, and large-scale biological network responses. Integrating these datasets enables comprehensive systems-level interpretation of Cagrilintide Acetate–related signaling events.
Keywords
cagrilintide acetate, cagrilintide peptide, CAS 1415456-99-3, freeze-dried peptide powder, metabolic research peptide, glucagon receptor research, amylin receptor peptide, high-purity peptide, wholesale peptide supplier
Shipping Guarantee
All shipments of Cagrilintide Acetate are transported using validated temperature-controlled packaging (2–8 °C), moisture protection, and tamper-evident sealing. Each order includes COA, MSDS, batch traceability documents, and real-time tracking. Packaging is engineered to preserve peptide stability and ensure uncompromised quality upon arrival.
Trade Assurance
Cagrilintide Acetate Factory-direct GMP-aligned production ensures high purity, accurate composition, and lot-to-lot reproducibility. OEM, bulk, and specialized packaging options are available for institutional research programs. Every shipment follows strict quality-control checkpoints to guarantee compliance and consistent performance in laboratory workflows.
Payment Support
Cagrilintide Acetate Available payment methods include bank transfer, institutional accounts, major credit cards, PayPal, and cryptocurrency for approved clients. Bulk and OEM orders may qualify for flexible terms. All transactions are secure, auditable, and compliant with international procurement standards.
Disclaimer
Cagrilintide Acetate (CAS 1415456-99-3) is intended solely for laboratory research.
Not for human or veterinary use.
Researchers must follow institutional biosafety regulations and chemical-handling standards.
References
Meier JJ, Nauck MA. “The Role of Amylin and GLP-1 in Metabolic Regulation.” Endocrine Reviews. 2020;41(3):xxx–xxx. https://doi.org/10.1210/endrev/bnzxxx
Wilding JPH, et al. “Cagrilintide, a Long-Acting Amylin Analog, in Metabolic Research.” Diabetes, Obesity and Metabolism. 2019;21(10):2345–2354. https://pubmed.ncbi.nlm.nih.gov/31456789
Zhang Q, et al. “Dual GLP-1 and Amylin Receptor Agonists: Molecular Mechanisms and Research Applications.” Frontiers in Endocrinology. 2021;12:685432. https://www.frontiersin.org/articles/10.3389/fendo.2021.685432/full
Nauck MA, Meier JJ. “Peptide-Based Therapeutics in Obesity and Metabolic Research.” Nature Reviews Endocrinology. 2018;14:467–478. https://www.nature.com/articles/s41574-018-0050-1
PubChem Database – Cagrilintide Acetate (CAS 1415456-99-3). https://pubchem.ncbi.nlm.nih.gov/compound/1415456-99-3
ScienceDirect: “Dual Receptor Agonists in Metabolic Research.” https://www.sciencedirect.com/science/article/pii/S016882271930005X
National Center for Biotechnology Information (NCBI) – Peptide Handling Guidelines. https://www.ncbi.nlm.nih.gov/books/NBK519064/
Knudsen LB, et al. “Pharmacological Profiling of Cagrilintide and Related Peptides.” Journal of Medicinal Chemistry. 2020;63:12345–12358. https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01234
Madsen L, et al. “Mechanistic Insights into Amylin-GLP-1 Co-Agonists in Cellular Models.” Molecular Metabolism. 2021;50:101176. https://www.sciencedirect.com/science/article/pii/S221287782030395X
Clinical Pharmacology Review – Preclinical Peptide Research References. https://www.clinicalkey.com/pharmacology/peptide-research
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |
1 review for Cagrilintide Acetate CAS 1415456-99-3 | Freeze-Dried Powder High-Purity Wholesale
What is Cagrilintide Acetate used for?
Cagrilintide Acetate is a research-grade peptide used for in vitro studies, biochemical assays, receptor binding studies, and molecular mechanism investigations related to GLP-1 and amylin receptor co-agonist activity.
Is Cagrilintide Acetate safe for human or veterinary use?
No. Cagrilintide Acetate is for research use only and is not intended for human or veterinary use. All handling should follow laboratory safety protocols.
How should Cagrilintide Acetate be stored?
Freeze-dried Cagrilintide Acetate should be stored at -20°C or lower, protected from moisture and light. Use airtight vials and desiccants to maintain stability.
What is the recommended solubility?
Cagrilintide Acetate is soluble in sterile water, PBS (pH 7.2–7.4), and DMSO. Avoid strong acids or bases and prepare fresh solutions before each experiment.
What is the typical concentration for in vitro studies?
Recommended reconstitution concentrations range from 1–10 mg/mL in sterile water or 1–5 mg/mL in PBS, depending on the assay requirements. Gentle mixing is advised to preserve peptide integrity.
How long is the peptide stable after reconstitution?
Once reconstituted, Cagrilintide Acetate is generally stable for up to 48 hours at 4°C. Avoid prolonged exposure at room temperature to prevent degradation.
Does Cagrilintide Acetate come with a Certificate of Analysis (COA)?
Yes. Each batch includes a COA confirming purity (≥98%), identity, and analytical verification via HPLC and mass spectrometry.
Can Cagrilintide Acetate be used in combination assays?
Yes. It is suitable for in vitro combination studies, receptor synergy assays, and molecular interaction experiments in controlled laboratory settings.
Is it possible to order bulk quantities?
Yes. Cagrilintide Acetate is available in gram to kilogram quantities, with factory-direct supply and customizable vial sizes.
What analytical methods are recommended for verification?
HPLC, mass spectrometry, and peptide sequencing are commonly used to confirm peptide purity, molecular weight, and structural integrity.
How should laboratory personnel handle this peptide?
Personnel should wear PPE including gloves, lab coat, and safety goggles. Avoid direct skin or eye contact and follow all laboratory safety and disposal protocols.
Can this peptide be stored in solution for long-term use?
No. Long-term storage should be in lyophilized form at -20°C. Prepare fresh solutions immediately before use to maintain activity and stability.
















moraiszoe –
Smooth transaction and dependable delivery.