Subtotal: $2.00
Capecitabine Factory wholesale
$7.00
Capecitabine (brand: Aimoga®) is an oral fluoropyrimidine prodrug that converts to 5-fluorouracil (5-FU) preferentially within tumors via thymidine phosphorylase. Ideal for preclinical research in breast, colorectal, and gastric cancer. Each box contains 12 tablets of 500?mg, manufactured by Chengdu Yuandong Biopharma. Approved under China NMPA H20203570 (product code 86902181000698; barcode 6950425900567).
?? For laboratory research use only.?Please consult the staff for other purposes?
描述
Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Capecitabine Tablets (Aimoga®) |
| Generic Name | Capecitabine |
| CAS Number | 154361?50?9 |
| Molecular Formula | C??H??FN?O? |
| Molecular Weight | ~359.3?g/mol |
| Dosage Form | Tablet for oral dosing |
| Strength | 0.5?g (500?mg) per tablet |
| Pack Size | 12 tablets per box |
| Approval Number | H20203570 (China NMPA) |
| Product Code | 86902181000698 |
| Manufacturer | Chengdu Yuandong Biopharmaceutical Co., Ltd. |
| Barcode | 6950425900567 |
| Storage | Store at room temperature; keep dry |
| Intended Use | Laboratory research only |
Mechanism of Action
Capecitabine is absorbed orally and metabolized via carboxylesterase and cytidine deaminase to 5′-deoxy-5-fluorouridine, then converted in tumor tissues to 5-FU by thymidine phosphorylase—leading to tumor-selective cytotoxicity.
cancer.gov+15pubmed.ncbi.nlm.nih.gov+15oncodaily.com+15
Research Applications & Pharmacology
-
Shows broader antitumor activity and higher tumor concentrations of 5-FU than IV 5-FU in xenograft models
-
Potent effects in breast, colorectal, gastric, and other solid tumor models
-
Rapid GI absorption; high PK variability; metabolic cascade involves dihydropyrimidine dehydrogenase (DPD), with genetic polymorphism affecting exposure
Safety Profile & Side Effects
Common laboratory and clinical-related effects include:
-
GI toxicity: diarrhea, nausea, vomiting, abdominal pain
-
Dermal: hand-foot syndrome, dermatitis
-
Hematologic: lymphopenia, anemia, neutropenia
-
Fatigue, hyperbilirubinemia
Interactions with warfarin, PPI, CYP2C9 inhibitors warrant caution
Lab handling: Use PPE, monitor enzyme and blood count markers in animal models
?? Research?Use Disclaimer
This product is strictly intended for laboratory research use only and not approved for clinical, therapeutic, diagnostic, or veterinary use. Misuse may cause serious health risks and compromise research outcomes.
其他信息
| 重量 | 1.1 公斤 |
|---|---|
| 尺寸 | 18 × 16 × 18 厘米 |

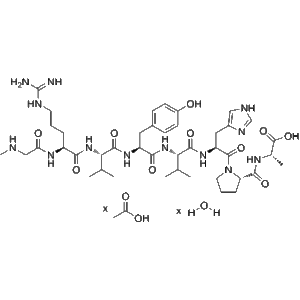
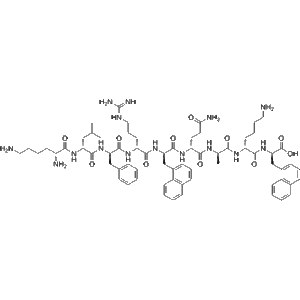
 Tilposide low price wholesale Research Peptide for Weight Loss and Metabolic Health
Tilposide low price wholesale Research Peptide for Weight Loss and Metabolic Health 






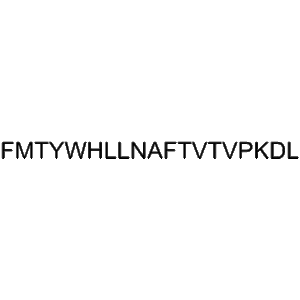
评价
目前还没有评价