No products in the cart.
Sale
Capecitabine | CAS 154361-50-9 | For research use only
Original price was: $56.00.$48.00Current price is: $48.00.
Capecitabine Tablets are an orally administered fluoropyrimidine prodrug that selectively converts to 5-fluorouracil within tumor tissues through a controlled enzymatic activation pathway. They are widely used in oncology research to study nucleic-acid synthesis inhibition, metabolic vulnerabilities, and tumor-specific cytotoxic responses.(For research use only. Not for human or veterinary use.)
Description
Product Description
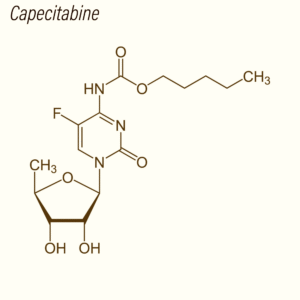
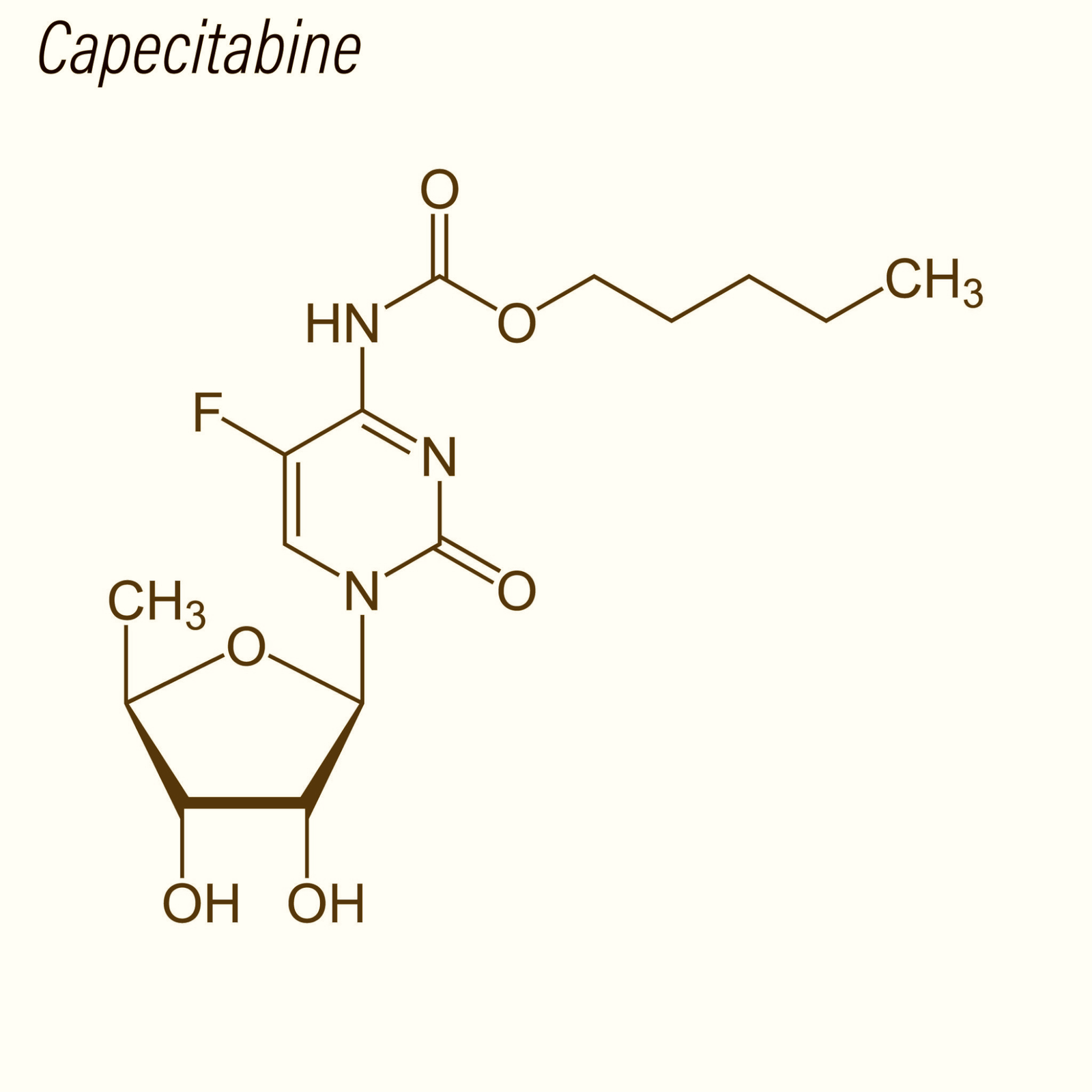
Capecitabine Tablets are a research-grade oral formulation of Capecitabine, an advanced fluoropyrimidine carbamate prodrug engineered for selective activation within tumor tissues. In biological systems, Capecitabine is converted into 5-fluorouracil (5-FU) through a three-step enzymatic cascade mediated by carboxylesterase, cytidine deaminase, and thymidine phosphorylase (TP). The elevated expression of TP in multiple solid tumors—including colorectal, gastric, and breast cancers—enables a tumor-targeted release profile that significantly enhances intratumoral 5-FU concentrations relative to systemic levels. This makes Capecitabine a powerful tool for studying localized cytotoxicity, DNA/RNA biosynthesis disruption, and thymidylate synthase inhibition.
For research laboratories, Capecitabine Tablets provide a highly controlled oral-dose model used in pharmacokinetic profiling, metabolic pathway mapping, and comparative studies between systemic and tumor-localized drug activation. The tablet form is particularly valuable for animal studies involving chronic oral dosing, drug-response modeling, therapeutic scheduling, and chemoresistance evolution. It offers improved dosing accuracy, batch consistency, and simplified administration compared with solution forms.
In mechanistic studies, Capecitabine enables detailed investigations of pyrimidine analog incorporation, RNA misprocessing, apoptosis signaling, and replication stress. Its role in modulating thymidylate synthase and altering nucleotide pools makes it essential for multi-omic workflows such as transcriptomics, metabolomics, and proteome-level stress-response mapping. Moreover, it is frequently incorporated into combination-therapy research involving DNA-damaging agents, immune modulators, anti-angiogenic compounds, and targeted inhibitors to biomimic clinical treatment regimens. With excellent chemical stability, predictable oral absorption, and well-defined metabolic pathways, Capecitabine Tablets serve as a reliable foundation for next-generation oncology research.
Product Specifications
| Parameter | Specification | Notes |
|---|---|---|
| Product Name | Capecitabine Tablets | Research-grade formulation |
| CAS Number | 154361-50-9 | Verified reference |
| Molecular Formula | C₁₅H₂₂FN₃O₆ | Parent compound |
| Form | Tablets | Stable oral-dosage form |
| Assay Purity | ≥99% (HPLC) | High-purity research level |
| Appearance | White to off-white tablets | Visual specification |
| Stability | Stable at room temperature | Store in sealed container |
| Storage Conditions | 2–8°C recommended | Protect from moisture/light |
| Solubility | Tablet for oral dosing; active dissolves in aqueous biological media | Suitable for in vivo use |
| Intended Use | Research only | Not for human administration |
| Packaging | Customizable counts | Bulk/wholesale supplied |
Mechanism of Action
Capecitabine functions as a rationally engineered, tumor-selective prodrug of 5-fluorouracil (5-FU), designed to maximize intratumoral cytotoxicity while minimizing systemic exposure. Its mechanism of action is defined by a precisely coordinated, multi-step metabolic activation pathway involving carboxylesterase, cytidine deaminase, and thymidine phosphorylase (TP)—an enzyme highly expressed within many solid tumors. This metabolic hierarchy creates a preferential release of 5-FU in cancer cells, enabling targeted disruption of DNA and RNA metabolism.
Once administered, Capecitabine is rapidly absorbed in the gastrointestinal tract and undergoes its first transformation in the liver, where carboxylesterase converts it into 5′-deoxy-5-fluorocytidine (5′-DFCR). This metabolite circulates systemically and is further processed by cytidine deaminase, an enzyme distributed in both liver tissue and tumor microenvironments, producing 5′-deoxy-5-fluorouridine (5′-DFUR). The final and most critical activation step occurs inside tumor cells, where thymidine phosphorylase (TP) converts 5′-DFUR into active 5-FU. Because TP levels are significantly elevated in many malignancies—including colorectal, breast, gastric, pancreatic, and head-and-neck tumors—Capecitabine demonstrates increased tumor selectivity compared with direct 5-FU administration.
Once generated, 5-FU undergoes intracellular anabolism, producing several active metabolites that interfere with essential cellular functions. One of the key metabolites, 5-fluoro-2′-deoxyuridine-5′-monophosphate (FdUMP), forms a stable ternary complex with thymidylate synthase (TS) and 5,10-methylenetetrahydrofolate. This irreversible inhibition of TS leads to depletion of deoxythymidine monophosphate (dTMP), an essential precursor for DNA synthesis. The resulting imbalance in dNTP pools triggers replication fork collapse, S-phase arrest, misincorporation events, and widespread DNA strand breaks. These combined effects create substantial replicative stress and apoptosis in rapidly proliferating cancer cells.
Simultaneously, another major metabolite, 5-fluorouridine triphosphate (FUTP), incorporates into nascent RNA, disrupting rRNA maturation, tRNA stability, and mRNA processing. This RNA-directed toxicity results in defective ribosome assembly, impaired protein translation, and activation of cellular stress pathways. A third metabolite, 5-fluoro-2′-deoxyuridine triphosphate (FdUTP), incorporates into DNA, further compounding genomic instability and promoting apoptotic signaling.
The dual DNA/RNA targeting nature of Capecitabine-derived 5-FU distinguishes it from many single-mechanism chemotherapeutic agents. In tumor microenvironments, the combination of TS inhibition, pyrimidine imbalance, RNA misprocessing, and DNA misincorporation activates downstream pathways such as p53 signaling, mitochondrial apoptotic cascades, ER stress responses, and autophagy modulation. Additionally, interferon-stimulated genes and immune-related stress signals may increase, contributing to immunomodulatory effects in tumor-host interactions.
Importantly, Capecitabine’s reliance on enzymatic activation means that tumor genotype, TP expression levels, folate availability, TP/DPD balance, and metabolic flux can significantly influence its efficacy. This makes Capecitabine an ideal compound for precision-oncology studies, AI-driven prediction of drug sensitivity, chemoresistance modeling, and multi-omic pathway integration.
Applications
Oncology Research
Capecitabine Tablets are extensively used in experimental oncology to study tumor-selective chemotherapy, intratumoral drug metabolism, and cellular response to pyrimidine analogs. Models of colorectal, gastric, breast, and pancreatic cancer frequently incorporate Capecitabine for pathway elucidation.
Chemoresistance & Combination Therapy Studies
Researchers employ Capecitabine to generate resistant cell lines and animal models. These are essential for identifying resistance mutations, metabolic rewiring, transporter modulation, and adaptive signaling in cancer cells.
Pharmacokinetics & Drug Metabolism
Capecitabine’s multi-step activation provides a clear enzymatic cascade ideal for metabolic pathway tracing. Radiolabeling, LC-MS/MS, and metabolomics are commonly used to quantify intermediate metabolites.
Multi-Omic Stress Response
As a DNA/RNA-targeting agent, Capecitabine is ideal for transcriptomic and proteomic profiling of replication stress, apoptosis, immune signaling, and metabolic adaptations.
Research Models
Research-grade Capecitabine Tablets are widely incorporated into diverse preclinical models designed to investigate oral fluoropyrimidine activation, tumor-specific metabolism, chemotherapeutic response, pharmacokinetics, chemoresistance, and multi-omic pathway regulation. Because Capecitabine is a prodrug requiring sequential enzymatic activation, it performs especially well in in vivo systems where tissue-specific enzyme expression mimics clinical conditions. Below are the principal categories of research models and the scientific rationale for their use.
1. Tumor Xenograft and PDX Models
Capecitabine is extensively utilized in human tumor xenograft and patient-derived xenograft (PDX) models to study tumor-selective 5-FU delivery. These systems exploit differences in thymidine phosphorylase (TP) expression across tumor types, enabling realistic assessment of Capecitabine’s activation efficiency. Researchers frequently use colorectal, breast, gastric, pancreatic, and head-and-neck cancer xenografts to evaluate tumor regression, molecular response signatures, and differential intratumoral 5-FU accumulation. PDX models, in particular, preserve patient-specific metabolic and genomic landscapes, allowing Capecitabine response profiling that closely aligns with precision oncology studies.
2. Genetically Engineered Mouse Models (GEMMs)
GEMMs provide powerful platforms for dissecting Capecitabine’s pharmacodynamics under defined genetic contexts. Tumors arising from engineered mutations—such as KRAS, TP53, APC, PI3K, and BRCA alterations—exhibit diverse metabolic states that influence Capecitabine activation and 5-FU vulnerability. These models are critical for studying how TP expression, nucleotide balance, replication stress signaling, and DNA-repair capacity affect prodrug conversion. GEMMs also support long-term oral dosing regimens that examine adaptive resistance, tumor progression, and microenvironmental effects.
3. Pharmacokinetic, ADME, and Bioavailability Models
Rodent pharmacokinetic models allow precise evaluation of absorption, distribution, metabolism, and excretion (ADME) dynamics after oral tablet administration. Capecitabine’s multi-step conversion—carboxylesterase activation in the liver, cytidine deamination in peripheral tissues, and TP-mediated activation in tumors—makes PK profiling essential for mechanistic interpretation. Tissue sampling, plasma LC-MS/MS quantification, radiolabel tracing, and metabolite mapping provide comprehensive datasets for PK modeling, dose optimization, and simulation of clinical therapeutic windows. Such models are also crucial for comparing tablets vs. solutions in bioavailability studies.
4. Chemoresistance Development Models
Long-term Capecitabine or intermediate metabolite exposure in both in vitro and in vivo systems produces acquired resistance models that mimic clinical treatment failure. Resistant tumors or cell populations often show altered thymidylate synthase activity, metabolic rewiring, increased DNA-repair capacity, or modified nucleotide salvage pathways. These models enable systematic characterization of resistance drivers, cross-resistance with other fluoropyrimidines, and evaluation of novel combination strategies. Transcriptomic, proteomic, and metabolomic profiling of resistant populations is frequently performed.
5. Syngeneic and Immune-Competent Models
To investigate immunomodulatory consequences of fluoropyrimidine exposure, Capecitabine is integrated into immune-competent mouse models such as CT26 or 4T1 systems. These models allow researchers to assess changes in antigen presentation, tumor immunogenicity, T-cell infiltration, cytokine signaling, and immune-related apoptosis pathways. They are particularly relevant for evaluating combination protocols involving immune checkpoint inhibitors or immunostimulatory agents.
6. Orthotopic Cancer Models
Orthotopic implantation models provide a more physiologically accurate environment for studying Capecitabine response, metastasis suppression, and drug distribution relative to primary tumor sites. Examples include orthotopic colorectal cancer (cecal wall), gastric cancer (stomach wall), and breast cancer (mammary fat pad). These models offer superior insight into prodrug activation under native vascularization, stromal interactions, and microenvironmental gradients.
7. Organoid and Ex Vivo Tissue Models
Human- or animal-derived tumor organoids increasingly serve as high-fidelity systems for evaluating Capecitabine metabolism and 5-FU–mediated cytotoxicity. Organoids retain patient-specific enzyme expression levels, mutation profiles, and epigenetic states, enabling accurate assessment of sensitivity or resistance. Ex vivo slices and microfluidic tumor-on-chip platforms further support mechanistic modeling of drug penetration, metabolic flux, and apoptotic response.
8. Toxicology and Off-Target Assessment Models
Researchers employ specialized toxicology models to evaluate Capecitabine’s systematic and tissue-specific effects, including GI tract stress, hematological suppression, liver enzyme perturbation, and metabolic shifts. These models help identify biomarkers of toxicity, evaluate safe dosing windows, and compare differential effects across species and tissue types. High-dose, chronic-dosing studies support mechanistic investigations into mitochondrial function, folate-cycle dynamics, and RNA-processing perturbation.
9. Metabolic Knockout / Enzyme-Deficient Models
Knockout mice lacking enzymes such as TP, cytidine deaminase, or dihydropyrimidine dehydrogenase (DPD) are utilized to dissect each step of Capecitabine metabolism. These models are instrumental in defining rate-limiting steps, mapping metabolic flux, and evaluating how enzyme deficiencies amplify or suppress 5-FU toxicity. They also support computational pharmacogenomics and AI-based prediction of metabolic phenotypes.
Experimental Design Considerations
Dosing Strategy
Tablet dosing enables consistent oral gavage or free-feeding administration. Researchers should calculate dosing based on animal body weight and validated oncology protocols.
Monitoring Endpoints
Key endpoints include intratumoral 5-FU concentration, thymidylate synthase activity, apoptosis markers, RNA incorporation, and DNA replication stress signatures.
Combination Studies
When combining with targeted therapies, immune modulators, or radiotherapy, sequencing and timing significantly impact outcomes and should be pilot-tested.
Laboratory Safety & Handling Guidelines
Handle tablets with gloves and eye protection.
Avoid dust generation when splitting or crushing tablets for research.
Dispose of waste through approved chemical-hazard channels.
Follow SDS and institutional safety policies for cytotoxic compounds.
Integration with Multi-Omic & Computational Studies
Integration of Capecitabine Tablets into multi-omic and computational oncology workflows enables deeper mechanistic annotation, predictive modeling, and precision-focused hypothesis generation. Because capecitabine is converted into 5-fluorouracil (5-FU) preferentially within tumor tissues, multi-omic analyses help map the molecular determinants of this tumor-selective activation and identify genomic vulnerabilities that may amplify or diminish therapeutic responses.
Genomic Profiling Approaches
Whole-genome sequencing (WGS) and whole-exome sequencing (WES) are routinely used to characterize mutational signatures associated with fluoropyrimidine responsiveness, such as variations in DPYD, TYMS, MTHFR, and UMPS. Integrating these datasets with capecitabine exposure studies helps identify gene–drug interactions that affect the efficiency of 5-FU generation or catabolism. CRISPR-based perturbation screens further allow researchers to discover synthetic-lethal combinations and resistance-driving gene knockouts with high throughput.
Transcriptomic and Single-Cell RNA-Seq Integration
Bulk RNA sequencing and single-cell RNA-seq provide insights into how capecitabine alters transcriptional programs within heterogeneous tumor microenvironments. Researchers often map differentially expressed genes related to nucleic-acid metabolism, DNA damage responses, cellular stress signaling, and interferon pathways. Single-cell modalities allow resolution of cell-type-specific sensitivity patterns, enabling identification of resistant subpopulations and adaptive transcriptional states induced by fluoropyrimidine stress.
Proteomic and Phosphoproteomic Layers
Mass-spectrometry-based proteomics, phosphoproteomics, and protein-interaction profiling are useful for identifying downstream pathway disruptions triggered by 5-FU incorporation into RNA/DNA. These datasets can reveal alterations in ribosomal assembly, cell-cycle progression, apoptotic pathways, and mismatch-repair proteins. When integrated with transcriptomic data, multi-layer network reconstruction enables validation of post-transcriptional regulatory events that influence cytotoxicity.
Metabolomic & Fluxomic Modeling
Because capecitabine’s metabolites directly interact with nucleotide pools, metabolomic profiling (LC-MS, GC-MS, NMR) is especially valuable. Researchers can quantify shifts in pyrimidine metabolism, triphosphate accumulation, anabolic versus catabolic flow, and the biochemical impact of enzyme polymorphisms. Fluxomics using isotopic tracing further enables kinetic modeling of capecitabine activation pathways and competition between endogenous nucleotide substrates and toxic fluorinated analogues.
Computational Modeling & AI-Driven Prediction
Machine learning (ML) and deep-learning frameworks are frequently applied to integrate multi-omic data, including mutational signatures, metabolic flux data, and transcriptomic remodeling, into predictive algorithms for drug sensitivity. Systems-biology models simulate capecitabine activation dynamics, enzyme saturation kinetics, and tumor-microenvironment influences (e.g., hypoxia-driven expression changes in thymidine phosphorylase). AI-based drug-response predictors also help identify biomarker panels that correlate strongly with capecitabine efficacy and toxicity in preclinical settings.
Network Biology & Pathway Reconstruction
Multi-omic integration supports holistic pathway mapping to determine how capecitabine perturbs global regulatory networks. Network-propagation methods highlight key hubs (e.g., TP53, MYC, E2F family proteins) impacted by 5-FU-mediated transcriptional interference. Combining proteomic, metabolomic, and phosphoproteomic signatures allows construction of dynamic models of apoptosis, autophagy, and immune-modulation cascades, aiding in the identification of potential combination-therapy targets.
Computational Pharmacology & PK/PD Simulation
In silico pharmacokinetic/pharmacodynamic (PK/PD) models simulate capecitabine absorption, enzymatic conversion rates, metabolite accumulation curves, and dose-dependent cytotoxicity. These tools are particularly useful when comparing tumor-specific activation profiles or optimizing dosing schedules in in vitro or in vivo research models. Multi-omic datasets enhance model accuracy by incorporating actual biological variation in enzyme expression and metabolic capacity.
Integration with Multi-Omic Biomarker Discovery
By overlaying multi-omic datasets, researchers can systematically identify biomarkers that predict response, resistance, or metabolic liability. Biomarker panels often include combinations of genomic variants, transcript ratios, metabolic patterns, and post-translational modifications. These multi-dimensional signatures are essential for precision-oncology research and facilitate objective evaluation of capecitabine-induced cytotoxic mechanisms.
Things to note
In research models, Capecitabine may induce dose-dependent gastrointestinal stress, hematological suppression, and metabolic alterations. Mice and rats may exhibit weight loss, decreased activity, or changes in liver enzymes due to systemic exposure and metabolite turnover. High-dose studies may reveal enhanced apoptosis in non-target proliferative tissues, reflecting 5-FU exposure. Some studies observe immune modulation, changes in inflammatory cytokines, or minor cardiovascular effects. Because activation is TP-dependent, toxicity patterns may vary significantly between tissues with different enzyme levels.
Keywords
Capecitabine Tablets, Capecitabine research chemical, 5-FU prodrug, tumor-targeted chemotherapy research, fluoropyrimidine analog, thymidylate synthase inhibition, colorectal cancer model, gastric cancer model, oral chemotherapeutic research, xenograft dosing, metabolic activation pathway.
Shipping Guarantee
All shipments are prepared using validated temperature-controlled packaging systems designed to preserve the molecular stability and purity of the product during transit. Each shipment includes moisture-resistant protective layers and shock-absorbing materials to prevent environmental or mechanical stress. Comprehensive tracking is provided from dispatch to delivery, ensuring full visibility for laboratories and procurement teams. Every batch is accompanied by documentation and quality verification to confirm compliance with storage and transport requirements.
Trade Assurance
Our trade assurance program ensures factory-direct supply with consistent batch quality and scalable bulk availability for institutional and industrial research needs. Each lot is supported by a full documentation package, including COA, MSDS, production records, and traceability data. Customers benefit from secure procurement workflows that minimize risk and maintain strict quality control at every stage. Guaranteed delivery timelines are provided to support long-term research planning and uninterrupted experimental operations.
Payment Support
We support major international payment channels to meet the procurement standards of global research institutions and distributors. Options include bank wire transfer, corporate and institutional credit payment, and secure online processing through compliant financial platforms. These flexible methods help streamline purchasing procedures and reduce administrative delays. Dedicated support is available to assist with invoices, quotations, and financial documentation as needed.
Disclaimer
This product is intended strictly for laboratory research use and is not approved for human or veterinary applications. It must not be administered, consumed, or used for diagnostic or therapeutic purposes under any circumstances. Researchers should handle the material in accordance with institutional safety protocols and relevant regulatory guidelines. By purchasing or using this product, the buyer acknowledges compliance with all applicable research-use restrictions.
References
5-Fluorouracil: Mechanisms of Action and Clinical Strategies — Nature Reviews Cancer. OUCI+1
Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients — PubMed / Cancer Chemotherapy and Pharmacology. PubMed
Pharmacology and therapeutic efficacy of capecitabine: focus on breast and colorectal cancer — PubMed review article. PubMed
Capecitabine and radiation therapy for advanced gastrointestinal malignancies — PubMed clinical-research article. PubMed
Acquired resistance to 5-fluorouracil via HSP90/Src-mediated increase in thymidylate synthase expression in colon cancer — Oncotarget. oncotarget.com
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 16 cm |
Q1: What is Capecitabine used for in research?
Capecitabine is a fluoropyrimidine prodrug widely used to study tumor-specific chemotherapy and DNA/RNA synthesis inhibition. Researchers utilize it in preclinical cancer models to investigate metabolic activation pathways, cytotoxicity, and resistance mechanisms. Its selective conversion to 5-fluorouracil in tumors enables precise modeling of therapeutic effects.
Q2: How does Capecitabine become active?
Capecitabine undergoes a three-step enzymatic conversion: first, carboxylesterase in the liver generates 5'-deoxy-5-fluorocytidine (5'-DFCR), then cytidine deaminase forms 5'-deoxy-5-fluorouridine (5'-DFUR), and finally, thymidine phosphorylase (TP) in tumor tissues converts it into active 5-fluorouracil (5-FU). This tumor-selective activation allows researchers to mimic clinical pharmacodynamics in vitro and in vivo.
Q3: Which cell lines are compatible with Capecitabine research?
Capecitabine is commonly used in ER+ breast cancer lines (MCF-7, T47D), colorectal cancer lines (HCT116, HT-29), and pancreatic cancer models (PANC-1, MiaPaCa-2). It is also suitable for primary tumor-derived cells and organoid cultures. Cell-type-specific TP expression influences conversion efficiency and cytotoxicity outcomes.
Q4: Can Capecitabine be used in animal models?
Yes, Capecitabine Tablets are often administered orally in rodent xenograft, PDX, and GEMM models. Researchers monitor tumor regression, 5-FU intratumoral accumulation, and systemic toxicity. Chronic dosing studies can also model acquired chemoresistance.
Q5: How should Capecitabine Tablets be stored?
Tablets should be kept in a sealed container at 2–8°C, protected from light and moisture. Proper storage preserves stability, purity, and enzymatic activation potential for extended research use. Avoid repeated exposure to humidity or high temperatures.
Q6: What are the typical in vitro concentrations?
Capecitabine is typically studied at 10 nM–100 μM in cell culture, depending on the model. Optimal dosing should balance effective 5-FU generation with minimal off-target cytotoxicity. Dose-response assays are recommended to define the most suitable concentration.
Q7: Can Capecitabine be used in combination studies?
Yes, Capecitabine is frequently combined with DNA-damaging agents, kinase inhibitors, mTOR inhibitors, or immunotherapies. Combination studies help evaluate synergistic effects, resistance pathways, and signaling crosstalk. Proper controls and sequential dosing are important for reproducible results.
Q8: How does Capecitabine affect tumor cells?
Once converted to 5-FU, Capecitabine disrupts thymidylate synthase, depletes dTMP pools, and induces S-phase arrest. FUTP and FdUTP metabolites incorporate into RNA and DNA, impairing translation and genomic integrity. These effects collectively trigger apoptosis and replication stress in tumor cells.
Q9: Is Capecitabine suitable for multi-omic studies?
Yes, Capecitabine is widely used in transcriptomics, proteomics, and metabolomics to study tumor-specific stress responses. Its well-characterized metabolic pathway enables precise mapping of pathway alterations and drug-response biomarkers. Integration with computational modeling supports predictive analyses of efficacy and resistance.
Q10: What safety precautions are necessary?
Handle Capecitabine in a fume hood with gloves, lab coat, and eye protection. Avoid inhalation, ingestion, or skin contact. Dispose of residues according to institutional chemical safety guidelines.
Q11: Can Capecitabine generate chemoresistant models?
Yes, chronic exposure in vitro or in vivo can produce Capecitabine-resistant cells or tumors. These models are useful for studying adaptive signaling, thymidylate synthase overexpression, and drug-efflux pathways. They also facilitate testing of combination therapies.
Q12: What animal species are recommended?
Rodents, particularly mice and rats, are preferred due to well-characterized tumor xenograft and PDX protocols. Non-rodent species are occasionally used for pharmacokinetic modeling. Dosing must be carefully adjusted based on body weight and study objectives.
Q13: How is pharmacokinetic monitoring performed?
Researchers measure Capecitabine and metabolite levels in plasma and tumor tissues using LC-MS/MS or radiolabeling techniques. Sampling at multiple time points allows calculation of absorption, distribution, metabolism, and elimination kinetics. This informs dose optimization and mechanistic interpretation.
Q14: Can Capecitabine be used in organoid models?
Yes, patient- or animal-derived tumor organoids are increasingly used to evaluate Capecitabine activation and cytotoxicity. Organoids retain TP expression patterns and tumor-specific metabolic signatures. They are valuable for personalized drug-response studies and high-throughput screening.











Reviews
There are no reviews yet.