No products in the cart.
Sale
Cetuximab NO1 100 mg/50 mL | High purity | Factory manufactured
Original price was: $3.00.$2.00Current price is: $2.00.
Cetuximab NO1 (100 mg/50 mL, Solution) is a high-purity monoclonal antibody solution manufactured under stringent factory-controlled conditions for in vitro laboratory research and molecular mechanism studies. Its solution format facilitates precise dosing, reproducible assay preparation, and integration into receptor-binding and signaling pathway research workflows.
Description
Contents
hide
Product Description
Cetuximab NO1 Solution is a recombinant monoclonal antibody reagent supplied at 100 mg per 50 mL for laboratory research. Designed exclusively for in vitro mechanistic studies, this high-purity solution enables researchers to examine EGFR-associated molecular interactions, receptor-ligand binding, and downstream signaling events with high reproducibility.
Manufactured in a controlled facility with comprehensive quality checks, each batch is verified for purity, concentration consistency, and functional integrity, ensuring reliable results across multiple in vitro assays. Its ready-to-use liquid format minimizes preparation variability, reduces experimental errors, and supports high-throughput screening, comparative analyses, and time-course studies.
Cetuximab NO1 is compatible with receptor interaction assays, biochemical characterization, and reconstructed signaling models, providing mechanistic insight into molecular binding dynamics, receptor modulation, and pathway-level responses. When combined with computational modeling, docking simulations, and multi-omic data integration, it serves as a powerful tool for comprehensive mechanistic investigations.
Each unit comes with batch traceability, analytical documentation, and a Certificate of Analysis, supporting reproducibility, quality verification, and laboratory compliance. Cetuximab NO1 is intended strictly for in vitro research, providing a stable, scalable, and precise antibody solution for molecular studies.
Product Specifications
| Parameter | Specification |
|---|---|
| Product Name | Cetuximab NO1 |
| Antibody Type | Recombinant monoclonal antibody |
| Form | Solution |
| Concentration | 100 mg / 50 mL |
| Purity | ≥99% (HPLC / analytical grade) |
| Appearance | Clear to slightly opalescent solution |
| Molecular Weight | ~145 kDa |
| Isotype | IgG1 |
| Target | EGFR (Epidermal Growth Factor Receptor) |
| Storage Conditions | 2–8°C, protected from light |
| Stability | Stable under recommended storage conditions |
| Buffer | Laboratory-grade aqueous formulation |
| Packaging | Sealed, research-grade container |
| Manufacturing Standard | Factory manufactured with QC and GMP-like procedures |
| Batch Traceability | Full batch identification and documentation available |
| Documentation | COA, analytical data, purity validation |
| Intended Use | In vitro laboratory research and molecular mechanism studies only |
| Supply Options | Single unit, bulk, wholesale |
| Application Compatibility | Compatible with receptor-binding assays, signaling pathway studies, multi-omic integration |
Technical Notes
Cetuximab NO1 Solution is provided as a ready-to-use liquid formulation, ensuring consistent concentration and reduced preparation variability for in vitro assays. The 100 mg / 50 mL total volume is suitable for extended mechanistic studies, multi-condition experiments, and comparative analyses without frequent resupply.
High analytical purity (≥99%) is confirmed using HPLC and other validated analytical methods, minimizing background interference in receptor interaction and signaling pathway studies. The solution is formulated in a laboratory-compatible buffer system, ensuring stability, solubility, and compatibility with biochemical assays, recombinant protein platforms, and high-throughput screening workflows.
Controlled storage conditions (2–8°C, light protection) help maintain antibody integrity, functional binding activity, and reproducibility. Each batch is supplied with Certificate of Analysis and complete documentation, providing traceability, quality verification, and support for regulatory-compliant laboratory research.
Overall, these specifications make Cetuximab NO1 Solution a versatile, stable, and reproducible reagent, ideal for EGFR mechanistic studies, receptor-binding characterization, and integrative multi-omic research in strictly controlled in vitro settings.
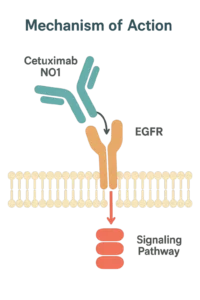
Mechanism of Action
Cetuximab NO1 is a recombinant monoclonal antibody that specifically binds to the extracellular domain of EGFR (Epidermal Growth Factor Receptor) in vitro, providing a robust tool for studying receptor-ligand interactions, conformational changes, and downstream signaling mechanisms. Its high affinity and solution-based formulation allow precise quantitative binding studies and mechanistic assays under controlled laboratory conditions.
In cell-free and recombinant protein systems, Cetuximab NO1 facilitates detailed analysis of binding kinetics, epitope mapping, and receptor dimerization events. Researchers can examine competitive binding behavior, receptor occupancy, and interaction specificity without confounding cellular complexity, enabling high-resolution insights into EGFR molecular mechanisms.
Within reconstituted in vitro signaling platforms, the antibody can be applied to investigate downstream molecular events, including signal propagation, phosphorylation cascades, and cross-talk between pathways. Controlled exposure in these models allows the dissection of dose-dependent effects on receptor activation and intracellular signaling networks, supporting mechanistic elucidation.
The solution format ensures consistent concentration delivery, reducing variability across experiments and enabling high-throughput or comparative analyses. This is critical for time-course studies and mechanistic modeling of EGFR-mediated molecular interactions.
Cetuximab NO1 is also suitable for integration with computational and structural biology approaches, including molecular docking, binding simulations, and in silico prediction of epitope interactions. Combining experimental and computational data enhances mechanistic interpretation, structure-function analysis, and pathway mapping.
Overall, Cetuximab NO1 serves as a versatile molecular tool for in vitro research, enabling researchers to investigate receptor-mediated signaling, molecular interactions, and EGFR-related pathway dynamics. Its high purity, validated activity, and solution-based format provide reproducible and reliable performance for detailed mechanistic studies in laboratory research environments.
Applications
Cetuximab NO1 Solution is widely applied in in vitro laboratory research as a molecular tool for studying EGFR-mediated signaling and receptor-ligand interactions. Its high purity and ready-to-use solution format facilitate precise experimental control, reproducibility, and integration across diverse mechanistic study platforms.
In receptor-binding studies, Cetuximab NO1 is used to examine EGFR occupancy, dimerization, and competitive ligand interactions in cell-free or recombinant protein systems. Researchers can quantify binding kinetics, epitope specificity, and interaction strength, providing detailed insight into molecular mechanisms underlying receptor regulation.
Within signal transduction research, Cetuximab NO1 supports investigation of downstream signaling events, such as phosphorylation cascades, secondary messenger modulation, and pathway cross-talk. The antibody enables controlled evaluation of dose- and time-dependent effects on intracellular signaling networks, assisting mechanistic dissection of EGFR-related pathways in reconstructed in vitro models.
Cetuximab NO1 is also suitable for biochemical and structural studies, including receptor conformation analysis, protein-protein interaction assays, and mechanistic characterization of ligand-induced molecular changes. Its stability and consistent formulation make it ideal for comparative studies, time-course experiments, and multi-condition assays.
In high-throughput screening (HTS) and assay development, the antibody serves as a reference reagent to validate assay sensitivity, specificity, and reproducibility. This enables robust benchmarking in diverse laboratory workflows and supports large-scale mechanistic investigations.
Additionally, Cetuximab NO1 can be combined with computational modeling, molecular docking, and in silico predictive studies, allowing researchers to integrate experimental data with structural and dynamic simulations. This synergy enhances mechanistic interpretation, pathway mapping, and structure-function relationship analysis.
Overall, Cetuximab NO1 Solution provides a versatile, reliable, and reproducible tool for EGFR receptor-binding analysis, signaling pathway investigation, and mechanistic molecular research, making it indispensable for in vitro laboratory studies that require precision and consistency.
Research Models
Cetuximab NO1 Solution is compatible with a wide range of in vitro research models designed to investigate EGFR receptor biology, molecular interactions, and signaling pathways under controlled laboratory conditions. Its high purity and solution format allow precise dosing, reproducibility, and integration across multiple experimental platforms.
In cell-free biochemical systems, Cetuximab NO1 enables detailed analysis of direct receptor-ligand interactions, binding kinetics, and competitive occupancy. These simplified models provide high-resolution mechanistic insights without the complexity of cellular environments, allowing researchers to isolate specific molecular events and interaction dynamics.
Recombinant protein and reconstituted signaling pathway platforms allow the study of downstream molecular mechanisms, including receptor dimerization, activation events, and intracellular signal propagation. These models are suitable for time-course experiments, dose-response analyses, and pathway mapping, providing a controlled environment to dissect EGFR-mediated signal transduction.
Cetuximab NO1 is also compatible with in vitro cellular models, such as immortalized or primary cell-derived systems, for mechanistic receptor signaling studies. Within these controlled environments, researchers can investigate receptor localization, phosphorylation dynamics, and pathway cross-talk, while strictly maintaining non-clinical, non-animal experimental conditions.
For high-throughput screening (HTS) applications, Cetuximab NO1 serves as a reference or control antibody, supporting assay validation, performance benchmarking, and reproducibility assessment. Its stability and consistent formulation make it suitable for multi-condition experiments and large-scale mechanistic studies.
Additionally, the antibody integrates seamlessly with computational and multi-omic research models, where experimental results can be correlated with in silico simulations, molecular docking studies, and proteomic or transcriptomic analyses. This hybrid approach enhances mechanistic interpretation and structural-functional analysis.
Overall, Cetuximab NO1 Solution provides a versatile and reliable reagent for in vitro research, enabling detailed mechanistic studies across biochemical, cellular, high-throughput, and computational models while maintaining reproducibility, precision, and laboratory safety.
Experimental Design Considerations
When utilizing Cetuximab NO1 Solution in in vitro mechanistic studies, careful experimental design is essential to ensure data accuracy, reproducibility, and mechanistic clarity. The solution’s high purity and ready-to-use format facilitate precise dose delivery and assay reproducibility, but validation of working concentrations is recommended for each experimental system.
Concentration-response assessments should be performed to define optimal ranges, preventing non-specific interactions and ensuring accurate interpretation of receptor-binding and signaling outcomes. Serial dilutions should be prepared using laboratory-grade buffers compatible with the assay platform to maintain chemical stability.
In all experimental setups, appropriate negative, vehicle, and reference controls are critical to distinguish specific EGFR-mediated effects from background variability. Replicate measurements and independent experimental runs enhance statistical robustness and confidence in observed results.
Environmental parameters such as temperature, pH, buffer composition, and incubation time must be standardized and documented. Even minor deviations can influence binding kinetics, receptor activation, and downstream pathway responses, particularly in sensitive biochemical or reconstituted signaling models.
Selection of assay formats should align with the research objective. Time-course assays provide insights into kinetic responses, pathway activation, and signal attenuation, while comparative designs enable cross-condition analysis. Integration of orthogonal analytical methods, such as proteomics, transcriptomics, or computational modeling, can support cross-validation and mechanistic interpretation.
Proper documentation of batch numbers, preparation protocols, and experimental conditions is recommended for reproducibility and audit readiness. When combined with multi-omic or computational approaches, these considerations allow for robust and mechanistically meaningful data generation.
By applying these design principles, researchers can maximize the analytical value and reliability of Cetuximab NO1 Solution in EGFR receptor-binding studies, signaling pathway exploration, and molecular mechanism research, ensuring reproducible and high-quality outcomes in strictly in vitro research contexts.
Laboratory Safety & Handling Guidelines
Cetuximab NO1 Solution is intended exclusively for laboratory research and in vitro studies. Handling should follow established chemical and biological safety protocols, ensuring a safe working environment while maintaining the integrity and reproducibility of experiments.
All personnel must wear appropriate personal protective equipment (PPE), including laboratory coats, gloves, and eye protection. Handling should be conducted in well-ventilated areas or designated laboratory safety cabinets to minimize potential exposure to aerosols or accidental spills.
Avoid direct contact with skin, eyes, and mucous membranes. In the event of accidental contact, rinse affected areas immediately with copious amounts of water and follow institutional reporting procedures. Spills should be contained and cleaned using approved laboratory decontamination procedures.
Cetuximab NO1 Solution should be stored at 2–8°C, in its original sealed container, and protected from direct light. Repeated temperature fluctuations or exposure to extreme conditions should be avoided to preserve antibody stability and functional integrity.
When preparing working solutions, use only laboratory-grade buffers and consumables to prevent contamination or degradation. Clearly label all secondary containers with product name, concentration, preparation date, and operator identification to maintain traceability and prevent misuse.
Unused solution, contaminated materials, and consumables must be disposed of according to institutional chemical and biological waste protocols, ensuring environmental and laboratory safety compliance.
Routine surface cleaning, equipment decontamination, and laboratory hygiene should be performed after each experiment to prevent cross-contamination and maintain reproducibility. Documentation of storage, handling, and disposal procedures supports reproducible research and audit readiness.
By following these laboratory safety and handling guidelines, researchers can maintain a safe and compliant laboratory environment, protect personnel, and ensure consistent, high-quality results when using Cetuximab NO1 Solution in strictly in vitro mechanistic studies.
Integration with Multi-Omic & Computational Studies
Cetuximab NO1 Solution is well-suited for integration into multi-omic research workflows to enable comprehensive mechanistic insights into EGFR signaling pathways. Its high purity and solution format allow consistent application across proteomic, transcriptomic, and metabolomic assays, supporting reproducible in vitro studies and enabling cross-platform correlation of molecular responses.
In proteomic studies, Cetuximab NO1 can be used to assess protein abundance, receptor interaction networks, and post-translational modifications associated with EGFR-mediated signaling. Quantitative proteomics can reveal dynamic changes in protein complexes, phosphorylation events, and interaction hubs, providing mechanistic insight into cellular pathways in a controlled in vitro environment.
For transcriptomic analyses, the reagent facilitates evaluation of gene expression profiles linked to receptor modulation and signaling pathway activity. Integration of transcriptomic data with proteomic observations allows researchers to identify regulatory networks, transcriptional responses, and downstream molecular effects associated with EGFR mechanistic events.
In metabolomic studies, Cetuximab NO1 supports analysis of pathway-specific metabolite changes, molecular flux, and biochemical alterations triggered by receptor engagement. Combining metabolomic data with proteomic and transcriptomic layers enhances understanding of molecular regulation, pathway crosstalk, and cellular adaptation mechanisms.
Beyond experimental omics, the antibody can be incorporated into computational and in silico models, including molecular docking, receptor-ligand binding simulations, and structural predictions. These approaches enable researchers to simulate interaction kinetics, predict binding conformations, and refine mechanistic hypotheses.
The integration of experimental multi-omic datasets with computational modeling provides a robust platform for cross-validation, pathway mapping, and structure-function analysis. Researchers can identify key regulatory nodes, molecular switches, and mechanistic bottlenecks in EGFR-mediated signaling.
Overall, Cetuximab NO1 Solution serves as a versatile reagent bridging experimental and computational approaches, supporting comprehensive, reproducible, and mechanistically rich in vitro research. This integration enables researchers to generate data-driven insights and predictive models for detailed molecular mechanism studies.
Things to Note
For laboratory research and in vitro use only
Not suitable for human or animal applications
Maintain cold-chain storage for stability
Verify batch documentation prior to use
Compatible with standard assay buffers and platforms
Keywords
Cetuximab NO1 solution, 100 mg/50 mL research antibody, high-purity EGFR antibody, in vitro receptor signaling study, molecular mechanism reagent, factory manufactured, low-price wholesale supply, laboratory research antibody, receptor-binding analysis, Tumor (compound) Research, multi-omic integration
Shipping Guarantee
Secure research-grade packaging ensures the chemical integrity and functional stability of Cetuximab NO1 Solution during transit. Temperature-controlled logistics are employed to prevent degradation and maintain consistent reagent performance. Global shipping supports laboratory delivery worldwide, allowing researchers to receive reagents intact, ready for immediate experimental use. All shipments are handled under strict tracking and quality verification protocols to ensure reliable delivery.
Trade Assurance
Factory-direct supply guarantees full batch traceability, documented quality control, and analytical verification for each unit of Cetuximab NO1 Solution. Bulk and wholesale procurement options are available to support long-term projects, high-throughput screening workflows, and multi-condition studies. Each batch is accompanied by COA and analytical documentation, ensuring reproducibility and compliance with laboratory research standards. This system provides researchers with consistent, high-quality reagents for mechanistic investigations.
Payment Support
Multiple secure payment methods are supported, including Credit Card, T/T (Telegraphic Transfer), and Cryptocurrency with encrypted payment protocols. These options provide flexibility and security for research institutions, commercial laboratories, and educational facilities. All transactions are processed through verified channels, ensuring safe, fast, and traceable procurement. This streamlined system allows efficient ordering and reliable delivery for laboratory research needs.
Disclaimer
Cetuximab NO1 Solution is intended exclusively for laboratory research and in vitro studies. It is not for human or animal use and must not be applied for clinical, diagnostic, or therapeutic purposes. Researchers are required to follow laboratory safety protocols, handling procedures, and storage guidelines. The product should be used solely for experimental investigation, ensuring reproducibility, compliance, and scientific integrity.
References
PubChem – Cetuximab
https://pubchem.ncbi.nlm.nih.gov/compound/CetuximabDrugBank – Cetuximab
https://go.drugbank.com/drugs/DB01269NCBI – Chemical & Protein Resources
https://www.ncbi.nlm.nih.gov/pccompound/?term=CetuximabRCSB Protein Data Bank – Cetuximab Structure
https://www.rcsb.org/ligand/CTXWikipedia – Cetuximab
https://en.wikipedia.org/wiki/Cetuximab
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 26 × 25 × 26 cm |
1 review for Cetuximab NO1 100 mg/50 mL | High purity | Factory manufactured
Q1: Can Cetuximab NO1 be used for in vitro receptor studies?
A1: Yes, it is specifically formulated for mechanistic EGFR signaling studies and receptor-binding assays.
Q2: Is it suitable for clinical or animal use?
A2: No, it is strictly for laboratory research and must not be used in humans or animals.
Q3: How should it be stored?
A3: Store at 2–8°C, protected from light, to preserve stability and function.
Q4: Is bulk supply available?
A4: Yes, factory-direct bulk and wholesale options are available.
Q5: Does each batch come with COA?
A5: Yes, including analytical verification and batch traceability.
Q6: Can it integrate with computational modeling?
A6: Yes, it can support docking simulations and predictive mechanistic studies.
Q7: How should unused material be disposed of?
A7: Follow institutional chemical and biohazard waste protocols.
Q8: Can it be used in multi-omic workflows?
A8: Yes, compatible with proteomic, transcriptomic, and metabolomic studies.
Q9: Is it compatible with high-throughput screening?
A9: Yes, suitable as reference or control in HTS platforms.
Q10: Primary applications?
A10: EGFR receptor-binding, signaling pathway analysis, mechanistic studies, assay validation.
Q11: Is the solution stable?
A11: Yes, under recommended storage conditions it maintains high purity and functional integrity.
Q12: Can it support structure–function studies?
A12: Yes, ideal for binding and conformational analysis.
















JOHNEKA –
Pago recibido vía PayPal, gracias.