No products in the cart.
Sale
Compound Fetal Liver Iron Ammonium Tablets | Iron Supplement for Laboratory and Preclinical Research
Original price was: $3.00.$2.00Current price is: $2.00.
Compound Fetal Liver Iron Ammonium Tablets are formulated as a rich source of bioavailable iron for laboratory and preclinical research. They are designed to support studies on iron metabolism, hematopoiesis, and iron-deficiency models, providing a reproducible and standardized formulation for in vitro and in vivo experiments.
Description
Contents
hide
Product Description
Compound Fetal Liver Iron Ammonium Tablets consist of iron salts derived from ammonium-ferric complexes and enriched fetal liver extracts. The formulation is optimized for bioavailability and stability, allowing for consistent delivery of iron in laboratory models.
The tablets provide a controlled source of elemental iron and other trace nutrients that are critical for erythropoiesis, hemoglobin synthesis, and red blood cell maturation. They are widely used in preclinical studies, including:
Models of iron-deficiency anemia;
Investigations of iron transport and absorption mechanisms;
Evaluation of erythropoietic factors and regulatory pathways;
Studies on oxidative stress associated with iron metabolism;
Nutritional and metabolic research related to iron homeostasis.
By using standardized tablets, researchers can ensure reproducibility across experimental cohorts and reduce variability in iron supplementation studies. These tablets are particularly valuable in mechanistic studies of iron-related disorders, testing therapeutic interventions, and analyzing hematological parameters.
Product Specifications
| Item | Details |
|---|---|
| Product Name | Compound Fetal Liver Iron Ammonium Tablets |
| Active Ingredient | Iron (Fe) in ammonium-ferric complex |
| Source | Fetal liver extract and synthetic iron salts |
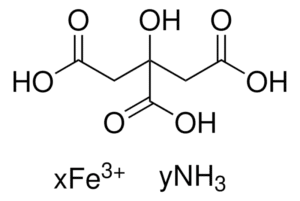
| Molecular Formula | Variable (mixture of iron compounds and amino acids) |
| Purity | ≥99% elemental iron equivalence |
| Appearance | Brownish tablet, uniform in size and weight |
| Solubility | Insoluble in water; dissolves in acidic medium |
| Storage Temperature | 2–25°C, dry and protected from light |
| Category | Iron supplement for research |
| Applications | Iron metabolism studies, anemia models, hematopoiesis research, oxidative stress assays |
| Formulation | Oral tablet for in vitro or animal studies |
| Stability | Stable under recommended storage conditions for 24 months |
| Shelf Life | 24 months |
| Supplier Type | Laboratory research chemical supplier |
| Intended Use | For laboratory and preclinical research only |
Mechanism of Action
Compound Fetal Liver Iron Ammonium Tablets provide bioavailable iron essential for hemoglobin synthesis, myoglobin formation, and cellular iron-dependent enzymatic processes.
1. Iron Absorption and Transport
Iron from these tablets is absorbed in the duodenum and jejunum through divalent metal transporter 1 (DMT1) and ferric reductase pathways. Once in circulation, iron binds to transferrin for systemic distribution to erythroid precursors and other iron-requiring tissues.
2. Erythropoiesis and Hemoglobin Synthesis
Supplemental iron supports red blood cell production by promoting heme synthesis in developing erythroblasts. This leads to increased hemoglobin concentration and improved oxygen-carrying capacity in experimental animal models.
3. Enzymatic and Metabolic Role
Iron acts as a cofactor in enzymes such as ribonucleotide reductase, cytochromes, and catalases, regulating DNA synthesis, oxidative metabolism, and cellular antioxidant defense. The tablets help maintain iron homeostasis in laboratory models.
4. Oxidative Stress and Iron Regulation
Iron supplementation must be carefully controlled to prevent free radical generation. Compound Fetal Liver Iron Ammonium Tablets provide a balanced iron source that minimizes pro-oxidant effects while maintaining physiological iron levels for research purposes.
5. Preclinical and Mechanistic Research Applications
Researchers use these tablets to simulate iron-deficiency and iron-repletion states in vitro and in vivo, study iron-responsive gene expression, test novel iron chelators, or evaluate nutritional interventions in animal models.
Side Effects
Compound Fetal Liver Iron Ammonium Tablets are intended strictly for laboratory and preclinical research. Considerations include:
Iron overload in sensitive models if doses exceed experimental requirements;
Potential oxidative stress at supra-physiological iron concentrations;
Gastrointestinal discomfort in animal models when administered orally;
Need for precise dosing and adherence to ethical research protocols.
These tablets are not intended for human consumption and should be handled under laboratory safety guidelines.
Keywords
Compound Fetal Liver Iron Ammonium Tablets, iron supplement, iron metabolism, anemia model, erythropoiesis research, hematology research, preclinical iron studies, high-purity iron tablets
Shipping Guarantee
All Compound Fetal Liver Iron Ammonium shipments are handled using validated logistics to preserve tablet integrity. Each package is sealed in moisture-proof containers with secondary protective wrapping. Products are shipped via express international couriers with full tracking and insurance coverage.
Trade Assurance
We Compound Fetal Liver Iron Ammonium ensure product authenticity, verified ≥99% elemental iron equivalence, and compliance with analytical standards. Each batch is supplied with a Certificate of Analysis (CoA). Our trade assurance policy guarantees replacement or refund for any deviation from listed specifications.
Payment Support
We Compound Fetal Liver Iron Ammonium provide flexible and secure global payment options to support international research transactions. Accepted payment methods include PayPal, major credit cards (Visa, MasterCard, American Express), telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are protected by industry-standard encryption and verified payment gateways to ensure confidentiality and fund security.
Disclaimer
All Compound Fetal Liver Iron Ammonium products listed are intended for laboratory and preclinical research use only and not for human or veterinary use. They are not drugs, medical devices, or diagnostics and should not be administered to humans or animals. Researchers must handle all materials in accordance with institutional biosafety and chemical safety guidelines. The information provided is for scientific reference only and does not imply therapeutic efficacy, safety, or regulatory approval.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
1. What are Compound Fetal Liver Iron Ammonium Tablets used for?
They are used in laboratory and preclinical research to study iron metabolism, anemia, and erythropoiesis.
2. Is this iron supplement safe for humans?
No, it is intended solely for laboratory research and preclinical studies.
3. How should the tablets be stored?
Store at 2–25°C, dry, and protected from light.
4. Can these tablets be used in animal models?
Yes, they are suitable for oral administration in animal studies with proper ethical approvals.
5. What is the iron content of each tablet?
Each tablet contains standardized ammonium iron equivalent to ≥99% elemental iron.
6. Can the tablets be dissolved for in vitro experiments?
They dissolve in acidic medium suitable for simulated gastrointestinal absorption studies.
7. Does the product include a Certificate of Analysis?
Yes, each batch is supplied with a CoA verifying purity and composition.
8. Are these tablets suitable for oxidative stress research?
Yes, they provide a controlled iron source to study iron-dependent oxidative pathways.
9. Can the tablets be combined with other supplements in research?
Yes, they can be used alongside other micronutrients for mechanistic studies.
10. What research applications are supported?
Iron-deficiency models, hematology studies, iron transport and absorption research, enzymatic studies, and preclinical nutritional experiments.
11. How is dosing determined for animal studies?
Doses should be calculated based on species, weight, and experimental design under ethical approval.
12. What safety precautions are needed?
Use laboratory PPE, follow biosafety protocols, and avoid ingestion by humans.
13. What type of iron is used in these tablets?
A combination of ammonium iron salts and fetal liver-derived iron compounds.
14. How long is the shelf life?
24 months under recommended storage conditions.
15. Who manufactures these tablets?
Supplied by certified laboratory research chemical manufacturers with standardized quality control.















Reviews
There are no reviews yet.