No products in the cart.
Cosentyx (Secukinumab) Injection 150?mg/mL Pre?filled Syringe – (For Research Use Only)
$1.00
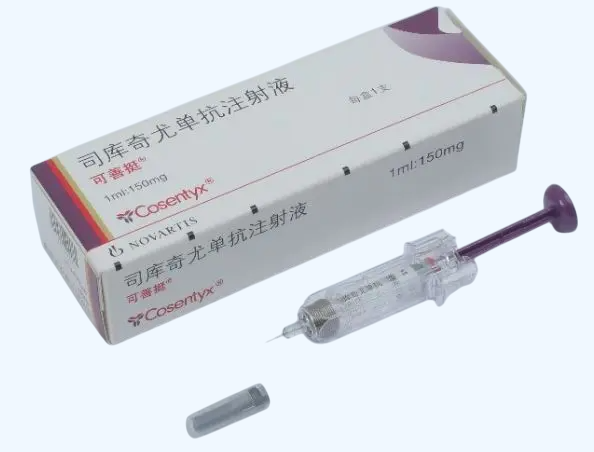
Cosentyx (secukinumab) is a human IgG1? monoclonal antibody formulated at 150?mg/mL in a pre?filled autoinjector. Produced by Novartis Pharma Stein AG under approval SJ20190023, this agent is ideal for laboratory research exploring IL?17A inhibition, cytokine-driven inflammation models, and autoimmune disease pathways. Wholesale and retail available. For research use only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
Cosentyx (secukinumab) binds and neutralizes interleukin?17A (IL?17A), a pro?inflammatory cytokine involved in psoriasis, psoriatic arthritis, and ankylosing spondylitis. By blocking IL?17A, secukinumab reduces inflammation and modulates immune signaling. Widely used in research to model autoimmune inflammation, IL?17 signaling dynamics, and test novel anti?cytokine therapies. Administered subcutaneously via 150?mg/mL pre?filled syringe or auto?injector. Packaged as individual syringes. Strictly for laboratory research use only.
Cosentyx (Secukinumab) Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Cosentyx (Secukinumab) Injection |
| Concentration | 150?mg/mL in 1?mL pre?filled syringe |
| Dosage Form | Subcutaneous injectable monoclonal antibody |
| Packaging | 1 syringe per box |
| Manufacturer | Novartis Pharma Stein AG |
| Approval Number | ???? SJ20190023 |
| Drug Standard Code | 86978679002666 |
| Barcode | Not yet assigned |
| CAS Number | 875356?43?7 (heavy chain), 875356?44?8 (light chain) |
| Molecular Type | Human IgG1? monoclonal antibody targeting IL?17A |
Cosentyx (Secukinumab) Mechanism of Action & Research Applications
Secukinumab is an IL?17A inhibitor that suppresses inflammation by blocking the cytokine signaling cascade mediated via Th17 cells. In research settings, Cosentyx is used to model:
Immune-mediated inflammation pathways
Psoriasiform skin, joint inflammation, and spondyloarthritis models
IL?17 signaling and cytokine modulation assays
Proof-of-concept studies for anti-cytokine therapies in vitro and in vivo
Cosentyx (Secukinumab) Side Effects (For Reference Only in Research Models)
Experimental analogs and clinical data suggest possible observations such as:
Upper respiratory infections, nasopharyngitis
Diarrhea, oral herpes, rash, or injection-site redness
Rare inflammatory bowel disease exacerbations
Anaphylaxis or hypersensitivity in latex-sensitive protocols
Monitor inflammatory markers and model tolerability parameters accordingly.
Disclaimer
Cosentyx (secukinumab) Injection is strictly intended for laboratory research use only. Not for human or veterinary therapeutic or diagnostic use.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 23 × 36 × 23 cm |











Reviews
There are no reviews yet.