No products in the cart.
Entecavir Dispersible Tablets 0.5?mg (He’en) – Low price Wholesale
$1.00
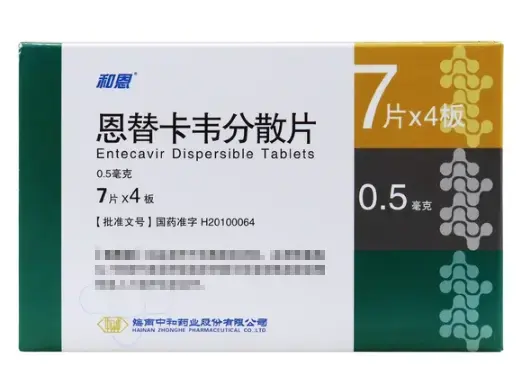
He’en’s Entecavir Dispersible Tablets feature 0.5?mg entecavir per tablet, packaged as 4 blisters of 7 tablets each (total 28). Manufactured by Hainan Zhonghe Pharmaceutical Co., Ltd., and approved under NMPA H20100064, this format dissolves easily in water, making it ideal for pediatric, in vitro, or formulation research. ?? For laboratory and research use only.?Please consult staff for other specifications and uses?
Description
Entecavir is a potent guanosine nucleoside analogue that inhibits hepatitis B virus (HBV) polymerase at multiple stages—blocking reverse transcription, priming, and DNA synthesis. The dispersible tablet ensures rapid administration, enabling flexible dosing in animal models and dissolution-based assays.
Entecavir Dispersible Tablets Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Entecavir Dispersible Tablets (He’en) |
| Generic Name | Entecavir |
| CAS Number | 142217?69?4 |
| Molecular Formula | C??H??N?O? |
| Molecular Weight | ~277.28?g/mol |
| Dosage Form | Dispersible tablet |
| Strength | 0.5?mg per tablet |
| Pack Size | 28 tablets (7 tablets × 4 blisters) |
| Approval No. | H20100064 |
| Drug Code | 86905842000471 |
| Manufacturer | Hainan Zhonghe Pharmaceutical Co., Ltd. |
| Barcode | 6927516988940 |
| Storage Conditions | Store sealed below 30?°C, protect from moisture |
| Intended Use | Laboratory research—HBV inhibition, pharmacokinetics, pediatric models |
Entecavir Dispersible Tablets Mechanism of Action
Entecavir competes with deoxyguanosine triphosphate to inhibit HBV polymerase, effectively halting virus replication by preventing priming, reverse transcription, and viral DNA synthesis. Its pharmacokinetic profile supports once-daily dosing, aided by a long half-life (128–149 hours).
Research Application Areas
HBV replication assays: Inhibition of viral polymerase function
Resistance mutation studies: Evaluating viral evasion mechanisms
Pharmacokinetics & bioequivalence: Including pediatric formulation comparisons
Pediatric and formulation research: Due to its dispersible nature
Combination therapy modeling: Integrase with other antivirals
Safety & Handling
Handling: Use in vitro or oral dosing in animal studies; employ standard PPE.
Storage: Keep sealed below 30?°C, in a dry environment.
Precautions: Not for clinical human use; monitor viral markers in prolonged studies.
Contraindications: Not intended for HBV-HIV co-infection without antiretroviral background.
Core Keywords
entecavir dispersible tablets, CAS 142217-69-4, H20100064 entecavir, HBV research tablet, pediatric antiviral tablet, lab-grade entecavir, wholesale antiviral tablets
Research Use Disclaimer
For laboratory and preclinical research only. Not approved for clinical, therapeutic, diagnostic, or veterinary applications. Misuse may pose legal or health risks. Use under institutional protocols.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 21 × 26 cm |











Reviews
There are no reviews yet.