Esubaglutide ? Injection 1?mg × 2 Prefilled Pens | YinuoQing by Intellivive Biotech (YinNuo Pharma)
$1.00
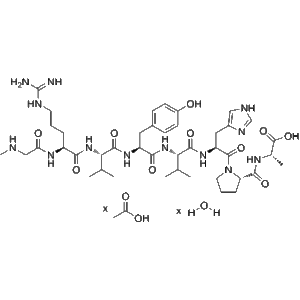
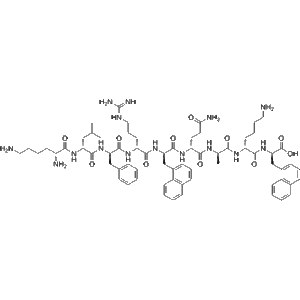
Esubaglutide ?, a next-generation ultra-long?acting GLP?1 receptor agonist, is delivered in two 0.5?ml (1?mg total) prefilled auto-injector pens per box. Produced by Intellivive Biotech (Suzhou) and commercialized under brand names YinuoQing/YinNuo Pharma, this advanced GLP-1RA is ideal for diabetes and metabolic research.
?? For research or analytical purposes only — Not for human consumption or therapeutic use.
描述
Esubaglutide ? Injection Description
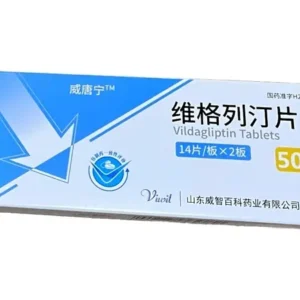
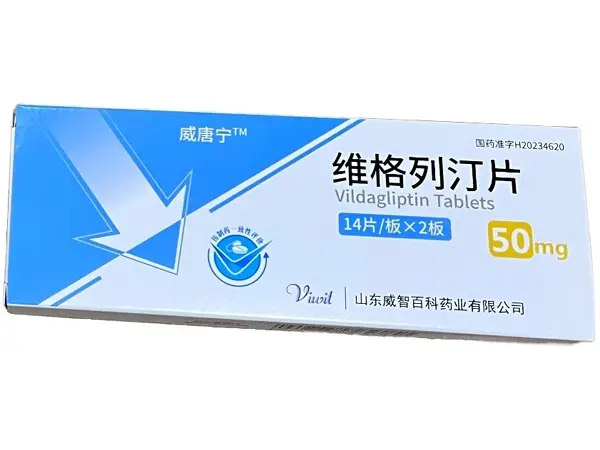
Product name: Esupagglutide ? injection (Yinoqing/Yinuo Pharmaceutical)
Packing specifications: 1mg (0.5ml)*2 (prefilled automatic injection pen) ? Product dosage form: injection ? Packaging unit: box
Approval number: National Medicine Standard S20250007 ? Drug code: 86984594000045
Manufacturer: Zhixiang Bio (Suzhou) Co., Ltd.
Product barcode: 6977560070016
Esubaglutide ? injection (marketed as YinuoQing®, also recognized by YinNuo Pharma) is China’s first domestically developed human?derived ultra?long?acting GLP?1 receptor agonist, designed for once-weekly subcutaneous administration in adult type 2 diabetes patients?.
-
Half-life: ~204 hours — among the longest globally for GLP?1 RAs?.
-
Clinical efficacy: Monotherapy for 4 weeks reduces HbA1c by ~1.1%; over 24 weeks, single-agent HbA1c reduction: 2.2%; with metformin, ~1.8%?.
-
Weight loss: Non-diabetic users lost ~4?kg after 4 weeks; 71% lost ?5% body weight?.
-
Cardiometabolic benefits: Reduced blood pressure (~4.1?mmHg), total and LDL cholesterol improvements?.
-
Convenience: Weekly dosing or even potentially biweekly, via easy-to-use injection pens?.
This product is a major milestone in metabolic therapeutic innovation, combining potent glycemic control, weight management, and user compliance with a single weekly injection?.
? Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Esubaglutide ? Injection (YinuoQing® / YinNuo Pharma) |
| Strength | 1?mg total (0.5?mg per 0.5?ml pen × 2 pens) |
| Dosage Form | Prefilled auto-injector pen |
| Packaging | Box containing 2 pens (0.5?ml each) |
| Approval Number | NMPA S20250007 |
| Drug Code | 86984594000045 |
| Manufacturer | Intellivive Biotech (Suzhou) |
| Barcode | 6977560070016 |
| Shelf Life | See packaging (standard for biologics) |
| Storage Conditions | Refrigerate at 2–8?°C; protect from light; do not freeze |
| Intended Use | Research and analytical applications only |
? Why Choose YinuoQing Esubaglutide ??
-
? Innovative biologic: China’s first human-derived, ultra-long-acting GLP?1 RA
-
? Extended half-life: Up to 204 hours for improved dosing compliance
-
? Strong clinical outcomes: Effective in HbA1c, weight loss, and cardiometabolic markers
-
? Lab-ready packaging: Precise dosing via prefilled pens, perfect for research
? Supporting Research & Market Context
-
NMPA Approval: January 26, 2025 — Diamond milestone for China’s diabetes therapy innovations?.
-
First Prescription Issued: February 2025 at Shanghai and Nanjing major hospitals?.
-
Global Status: Third human-derived long-acting GLP?1 RA with domestic IP (behind USA’s dulaglutide and tirzepatide)?.
-
Clinical Benefits: Notable HbA1c reduction, weight management, and metabolic risk improvements?zh.wikipedia.org.
-
Semaglutide (Ozempic®) Pre-filled Pens
-
Dulaglutide (Trulicity®) Autoinjectors
-
Tirzepatide (Mounjaro®) Dual GIP/GLP?1 Agonist Pens
? Final Summary
YinuoQing Esubaglutide ? Injection (1?mg, 2 pens) is a powerful research-grade GLP?1 receptor agonist with a record-breaking half-life, strong metabolic efficacy, and lab-friendly auto-injector pens. Fully traceable, clinically supported, and NMPA-approved, it’s ideal for research involving diabetes, obesity, and metabolic health interventions.
?? Reminder: strictly for laboratory or analytical use only — not for human use.
其他信息
| 重量 | 1.1 公斤 |
|---|---|
| 尺寸 | 26 × 32 × 24 厘米 |








djabri –
I have received the package. It took 11 days, which is a bit long. But thank you anyway.