No products in the cart.
Sale
F9170 TFA – Amphipathic Anti-HIV Peptide for Experimental Research
Original price was: $18.00.$16.00Current price is: $16.00.
F9170 TFA is an amphipathic peptide with strong anti-HIV activity. It inactivates HIV-1 virions by disrupting the viral membrane and targets the conserved cytoplasmic tail of HIV-1 env. Capable of crossing the blood-brain barrier, suitable for experimental virology and antiviral research. Laboratory use only.
Description
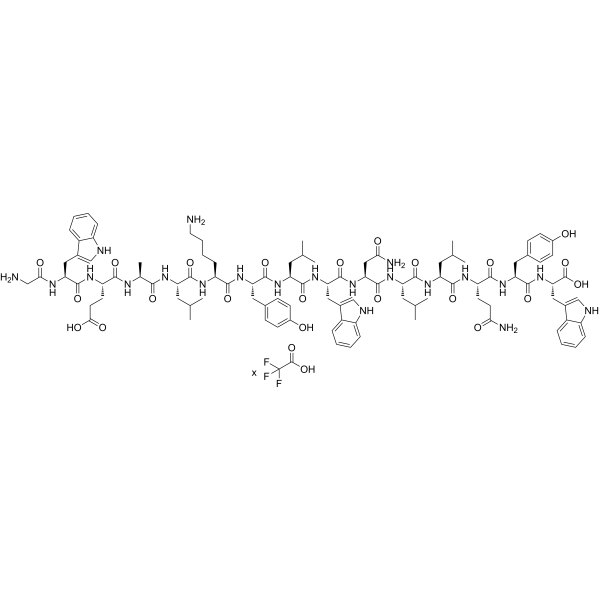
F9170 TFA is a synthetic amphipathic peptide exhibiting potent antiviral activity against HIV-1. With a purity of 98.68% (HPLC) and GMP manufacturing, this peptide is intended for experimental laboratory research in virology, neurovirology, and antiviral drug discovery. The amphipathic structure of F9170 TFA enables it to interact with viral membranes and disrupt their integrity, effectively inactivating HIV-1 virions.
Mechanistically, F9170 TFA selectively binds to the conserved cytoplasmic tail of the HIV-1 envelope glycoprotein (env), a region essential for viral assembly, maturation, and infectivity. By targeting this domain, F9170 TFA destabilizes the viral envelope, leading to virion inactivation and impaired infection in susceptible host cells. The peptide’s amphipathic nature allows insertion into lipid bilayers, further compromising the viral membrane and preventing the fusion of virions with target cells.
F9170 TFA also demonstrates the remarkable ability to cross the blood-brain barrier (BBB), which is critical for studies of HIV-1 neuropathogenesis. HIV-1 infection of the central nervous system (CNS) contributes to neurocognitive disorders in infected individuals, and peptides capable of reaching the CNS are valuable tools for mechanistic studies and the development of antiviral therapeutics. In laboratory research, F9170 TFA can be used to model virion inactivation, test combinational antiviral strategies, and explore peptide-based interventions for HIV-1 reservoirs in CNS compartments.
Its stability in TFA salt form ensures reproducible activity in vitro and in vivo experimental assays. Researchers can utilize F9170 TFA in various model systems, including primary human T cells, monocyte-derived macrophages, and CNS cell culture models. The peptide’s ability to penetrate cellular membranes further expands its utility for mechanistic studies of intracellular viral inhibition and antiviral peptide delivery strategies.
Beyond direct HIV-1 inactivation, F9170 TFA is a valuable tool for studying viral assembly, membrane fusion, and the functional roles of the cytoplasmic tail in env-mediated infectivity. It can also serve as a reference compound in high-throughput screening assays, mechanistic studies, and proof-of-concept experiments for novel antiviral agents. Its precise targeting minimizes off-target effects, making it a safe and reliable peptide for laboratory research when handled under controlled conditions.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | F9170 TFA |
| Synonyms | Amphipathic anti-HIV peptide |
| CAS Number | Not assigned |
| Molecular Type | Synthetic peptide |
| Purity | 98.68% (HPLC) |
| Appearance | Lyophilized peptide powder |
| Stability | Stable at -20°C; avoid repeated freeze-thaw cycles |
| Storage | Store at -20°C under inert atmosphere |
| GMP Compliance | Manufactured in GMP-certified facility |
| Application | Laboratory research in antiviral therapy, HIV-1 inactivation, CNS antiviral studies |
| Availability | Wholesale & retail supply |
Mechanism of Action & Research Applications
F9170 TFA’s antiviral mechanism is multi-faceted. Primarily, it binds to the conserved cytoplasmic tail of HIV-1 env glycoprotein, a crucial determinant for virion assembly, envelope incorporation, and viral infectivity. This binding disrupts proper env folding and trafficking, impairing viral maturation. Simultaneously, the amphipathic peptide integrates into the viral lipid membrane, creating local destabilization and enhanced membrane permeability. This dual mechanism—targeted protein binding plus membrane disruption—leads to effective inactivation of HIV-1 particles before they can infect host cells.
A distinctive feature of F9170 TFA is its ability to cross the blood-brain barrier. CNS reservoirs of HIV-1 present a significant challenge in antiretroviral therapy, and peptides capable of BBB penetration provide experimental models to investigate therapeutic delivery and viral suppression in neural tissues. Laboratory studies utilizing F9170 TFA can explore CNS-targeted viral inactivation, neuroprotection strategies, and antiviral peptide transport mechanisms.
F9170 TFA is also used in mechanistic studies of viral membrane biology, including investigations into viral assembly, fusion, budding, and interactions with host cell membranes. Its amphipathic nature allows researchers to probe membrane-associated processes critical for viral propagation. The peptide is suitable for in vitro assays using T cells, macrophages, and microglial cultures, as well as in vivo models that examine systemic and CNS antiviral effects.
Furthermore, F9170 TFA provides a valuable tool for combination studies with other antiviral agents, including reverse transcriptase inhibitors, protease inhibitors, and integrase inhibitors. By pairing F9170 TFA with established antiretrovirals, researchers can model synergistic effects, evaluate resistance mechanisms, and optimize experimental strategies for viral suppression.
The peptide’s high purity and GMP-compliant production ensure consistent and reproducible results, essential for preclinical studies and mechanistic investigations. Its precise targeting of the env cytoplasmic tail reduces nonspecific interactions, allowing focused research on HIV-1 viral inactivation and membrane disruption. F9170 TFA also serves as a reference molecule for developing new amphipathic peptides with antiviral potential or BBB penetration capabilities.

Side Effects (For Reference in Models)
In experimental studies, F9170 TFA may exhibit mild cytotoxicity at high concentrations, particularly in primary immune cells. Transient alterations in cell membrane integrity may occur due to its amphipathic and membrane-disrupting properties. No systemic toxicity has been observed in in vitro or controlled in vivo laboratory models at typical experimental doses. Researchers should handle the peptide according to institutional safety protocols, and standard protective measures should be applied to prevent unintended exposure.
Disclaimer
F9170 TFA is intended for laboratory research purposes only. It is not for human or veterinary use, diagnostic procedures, or clinical applications. Users must follow institutional safety protocols and local regulations when handling this peptide.
Keywords
F9170 TFA, anti-HIV peptide, amphipathic antiviral peptide, HIV-1 virion inactivation, env cytoplasmic tail targeting, blood-brain barrier peptide, laboratory antiviral research, synthetic HIV peptide, GMP peptide for research, CNS antiviral peptide, HIV experimental peptide, peptide antiviral therapy, wholesale peptide supplier, research peptide TFA, peptide for virology studies
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 53 × 26 × 53 cm |
What is F9170 TFA used for?
F9170 TFA is used for experimental research to inactivate HIV-1 virions and study antiviral mechanisms in vitro and in vivo. Laboratory use only.
How does F9170 TFA inactivate HIV-1?
It binds to the env cytoplasmic tail and disrupts the viral membrane, preventing infection.
Can F9170 TFA cross the blood-brain barrier?
Yes, it can penetrate the BBB, making it suitable for neurovirology research.
What is the purity of F9170 TFA?
The peptide is 98.68% pure by HPLC.
Is F9170 TFA GMP-compliant?
Yes, it is produced in a GMP-certified facility for research use.
Can F9170 TFA be used in combination with other antiviral agents?
Yes, it is suitable for combinational studies with reverse transcriptase, protease, or integrase inhibitors.
How should F9170 TFA be stored?
Store lyophilized powder at -20°C under an inert atmosphere.
Is F9170 TFA toxic to cells?
At high concentrations, it may cause mild cytotoxicity due to membrane disruption; standard laboratory safety is recommended.
Can it be used in animal models?
Yes, under controlled laboratory conditions, it is suitable for in vivo antiviral studies.
Does F9170 TFA affect viral assembly?
Yes, it disrupts the env cytoplasmic tail, impairing virion assembly and maturation.
Can it be used to study HIV-1 CNS infection?
Yes, its BBB penetration allows research on CNS viral reservoirs.
Is F9170 TFA orally active?
No, it is administered via experimental delivery methods in lab studies.
What cell types can be used with F9170 TFA?
Primary T cells, macrophages, microglia, and cell lines used for HIV-1 studies.
Can F9170 TFA serve as a reference peptide in assays?
Yes, it is suitable as a standard in antiviral screening and mechanistic studies.
Does F9170 TFA have off-target effects?
Its design minimizes off-target interactions, focusing on env cytoplasmic tail and membrane disruption.
Is it soluble in aqueous solutions?
Yes, it is soluble in water and buffer systems suitable for laboratory experiments.
Can F9170 TFA be used in high-throughput screening?
Yes, its reproducible activity makes it suitable for screening antiviral compounds.
Does F9170 TFA affect other viruses?
It is primarily designed for HIV-1; other virus studies require experimental validation.
What research fields use F9170 TFA?
Virology, antiviral drug discovery, neurovirology, peptide biology, HIV research.
Is F9170 TFA intended for human use?
No, it is strictly for experimental laboratory research purposes.













Reviews
There are no reviews yet.