No products in the cart.
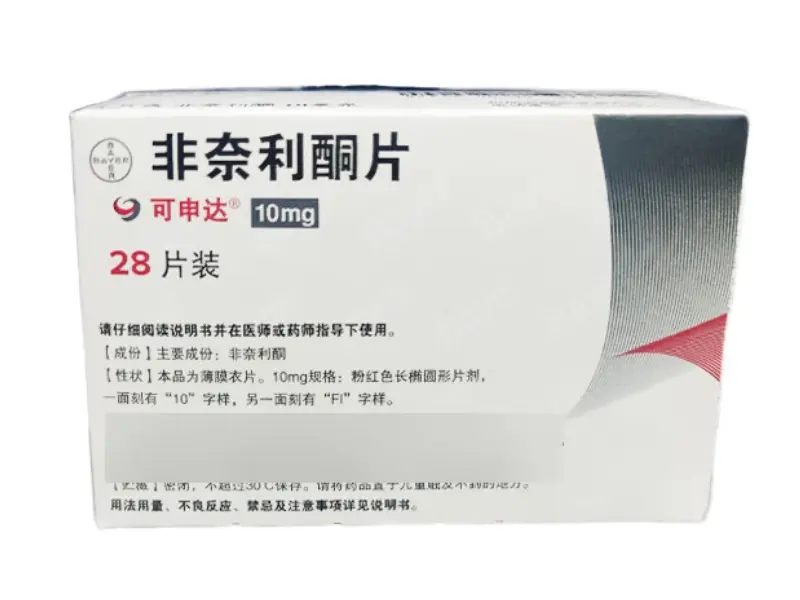
Finerenone Tablets
$2.00
Finerenone 10mg tablets (28 per box), branded as Kerendia by Bayer AG, are approved for research into chronic kidney disease and cardiovascular protection in Type2 diabetes. NMPA approved (HJ20220057) and globally recognized.
For laboratory research use only – not for human consumption or therapeutic use.
Description
Finerenone Tablets Description
Product name: Finerenone tablets (Keshenda)
Packaging specifications: 10mg*28 tablets Product dosage form: tablets Packaging unit: box
Approval number: National Medicine Standard HJ20220057 Drug standard code: 86978262000420
Manufacturer: Bayer AG, Germany
Product barcode: 6924147600869
Finerenone is a novel, non-steroidal, and selective mineralocorticoid receptor antagonist (MRA) approved for use in adults with chronic kidney disease associated with Type 2 diabetes. Clinical trials show it can significantly reduce the risk of kidney failure, cardiovascular death, and hospitalization due to heart failure.
First approved in China in June 2022 under NMPA number HJ20220057, Kerendia offers a powerful new treatment option with dual renal and cardiac protection. It was also added to the national reimbursement list in January 2023, improving patient accessibility.
Product Specifications
| Feature | Details |
|---|---|
| Product Name | Finerenone Tablets (Kerendia) |
| Strength | 10mg per tablet |
| Quantity | 28 film-coated tablets per box |
| Dosage Form | Oral tablets (film-coated) |
| Manufacturer | Bayer AG (Germany) |
| Approval Number (NMPA) | HJ20220057 (10mg) |
| Product Code | 86978262000420 |
| Barcode | 6924147600869 |
| Shelf Life | 36 months |
| Storage Conditions | Store sealed, under <30°C, away from children |
| Packaging | PVC/PVDC-aluminum blister packs |
Mechanism of Action & Clinical Highlights
Selective MRA: Blocks mineralocorticoid receptors in kidneys, heart, and blood vessels, reducing inflammation and fibrosis zh.wikipedia.org
Renal protection: FIDELIO-DKD trial showed an 18% reduction in composite renal endpoints.
Cardiovascular benefit: 14% reduction in cardiovascular events in CKD patients with T2D.
Hyperkalemia risk: Up to 18.3% incidence—requires serum potassium monitoring.
Blood pressure effect: Modest decreases in systolic (~2–4mmHg) / diastolic (~1–2mmHg) readings.
Why Choose Kerendia Finerenone?
First-in-class MRA with strong evidence in CKD + T2D
Backed by global RCTs and Chinese clinical data
Available in research-ready blister format
Ideal for metabolic, nephrology, and cardiovascular research
Spironolactone / Eplerenone (Steroidal MRAs)
SGLT 2 inhibitors (e.g., Dapagliflozin)
GLP-1 RAs (e.g., Semaglutide, Liraglutide)
Summary
Kerendia (Finerenone 10mg) is a high-quality, research-focused MRA with robust renal and cardiovascular evidence. Manufactured by Bayer, NMPA-approved, and blister-packed for lab use.
Reminder: strictly for laboratory research or analytical use only — not for human consumption or therapy.
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 18 × 16 cm |











Reviews
There are no reviews yet.