No products in the cart.
Sale
Human Angiotensin II (CAS 4474-91-3) – High-Purity Peptide Powder | Factory Manufacturing & Low-Cost Wholesale Supply
Original price was: $36.00.$28.00Current price is: $28.00.
Human Angiotensin II (CAS 4474-91-3) is a highly pure, research-grade octapeptide widely used in cardiovascular, renal, endocrine, and vascular biology studies. Produced through factory-direct GMP manufacturing, it is available in bulk quantities with full COA, MSDS, and QC documentation for reliable laboratory research.
Description
Contents
hide
Product Description
Human Angiotensin II (CAS 4474-91-3) is a potent endogenous octapeptide that plays a central role in the renin–angiotensin–aldosterone system (RAAS) and is one of the most widely studied bioactive molecules in cardiovascular and renal physiology. This research-grade peptide is supplied in high-purity lyophilized powder format and is designed exclusively for laboratory applications requiring precise biochemical, receptor-binding, and physiological characterization. Due to its strong vasoconstrictive activity and regulatory influence on blood pressure, electrolyte balance, and fluid homeostasis, Human Angiotensin II serves as a foundational tool across multiple areas of biomedical research.
Produced using GMP-compliant solid-phase peptide synthesis (SPPS), this peptide meets stringent quality and purity requirements, ensuring consistent biological activity across experiments. Each batch undergoes rigorous characterization, including HPLC purity testing, mass spectrometry validation, and microbial/endotoxin screening, providing researchers with confidence in reproducibility and experimental accuracy. The lyophilized format enhances stability, simplifies long-term storage, and supports precise reconstitution for in vitro and in vivo studies.
Human Angiotensin II is widely utilized to activate AT1 and AT2 receptors, allowing detailed analysis of cellular signaling pathways, vascular smooth muscle contraction, oxidative stress responses, endocrine regulation, inflammation, and hypertensive mechanisms. Its well-defined pharmacological profile makes it suitable for a broad range of research categories, including cardiovascular disease models, renal function studies, adrenal hormone secretion assays, metabolic regulation, and multi-omic system-level investigations.
Factory manufacturing ensures streamlined production, cost efficiency, and flexible customization options for OEM and bulk orders. High-volume synthesis capabilities support academic research programs, biotechnology companies, CROs, and pharmaceutical discovery pipelines. Each order includes essential documentation—COA, MSDS, QC release forms, and batch traceability—ensuring reproducibility and regulatory alignment for institutional laboratories.
With its strong relevance to hypertension, fibrosis, nephrology, endocrine disorders, and vascular pharmacology, Human Angiotensin II remains an indispensable reagent in experimental medicine. Our high-purity, factory-direct supply provides researchers with reliable peptide quality at competitive wholesale pricing, making it an ideal choice for both routine and advanced scientific investigations.

Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Human Angiotensin II |
| CAS Number | 4474-91-3 |
| Molecular Formula | C<sub>50</sub>H<sub>71</sub>N<sub>13</sub>O<sub>12</sub> |
| Molecular Weight | 1046.2 g/mol |
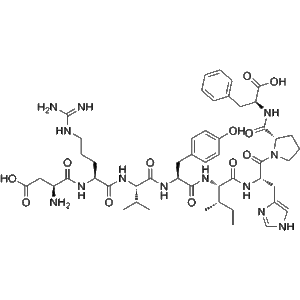
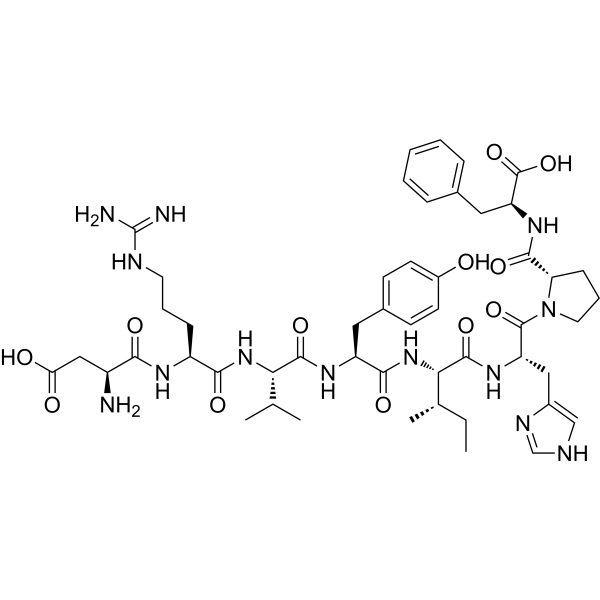
| Sequence | Asp-Arg-Val-Tyr-Ile-His-Pro-Phe (DRVYIHPF) |
| Purity | ≥98% (HPLC) |
| Form | Lyophilized Peptide Powder |
| Appearance | White to off-white powder |
| Solubility | Soluble in sterile water, PBS, or suitable buffers |
| Storage Temperature | 2–8 °C (lyophilized); −20 °C for long-term stability |
| Stability | Stable for 24 months when stored properly |
| Endotoxin Level | <0.1 EU/mg |
| Source | Synthetic (GMP-grade SPPS) |
| Packaging Options | 1 mg, 5 mg, 10 mg, customized OEM bulk |
Notes :
Human Angiotensin II is manufactured under GMP-aligned peptide synthesis conditions to ensure exceptional batch-to-batch reproducibility and research-grade purity. Every lot is validated through HPLC chromatographic profiling and mass spectrometric identity confirmation, ensuring that the peptide meets or exceeds industry standards for high-purity reagents. Lyophilization enhances structural stability, protects against hydrolysis, and enables convenient long-term storage at 2–8 °C, while reconstitution immediately before use ensures optimal activity in both cell-based and animal studies.
Sterility and endotoxin levels are tightly controlled to support sensitive applications, including vascular tissue assays, renal perfusion studies, receptor activation profiling, and endocrine system models. Packaging options are flexible, ranging from small laboratory-scale vials to large OEM bulk lots suitable for biotechnology and pharmaceutical research pipelines. Each batch is accompanied by complete documentation—including Certificate of Analysis, MSDS, QC report, and traceable batch identifiers—to meet institutional and regulatory quality requirements.
This high stability and purity profile makes Human Angiotensin II ideal for advanced mechanistic studies, high-throughput screening, and multi-omic integration workflows. Researchers can depend on consistent, validated performance across a broad range of experimental designs, from mechanistic receptor studies to whole-system physiological investigations.
Mechanism of Action
Human Angiotensin II is an endogenous octapeptide hormone that plays a central regulatory role in the renin–angiotensin–aldosterone system (RAAS), governing blood pressure, vascular tone, fluid balance, and endocrine responses. Its mechanism of action begins with high-affinity binding to the angiotensin II type 1 receptor (AT1R), a G protein–coupled receptor (GPCR) expressed throughout vascular smooth muscle, the kidney, adrenal cortex, heart, and central nervous system. Upon receptor activation, AT1R undergoes a conformational change that triggers Gq/11-mediated signaling, stimulating phospholipase C (PLC) and generating the second messengers IP₃ and DAG. These second messengers elevate intracellular Ca²⁺ levels and activate protein kinase C (PKC), resulting in potent vasoconstriction and modulation of downstream contractile and transcriptional programs.
Beyond classical vasoconstrictive activity, Angiotensin II also activates MAPK/ERK, JAK/STAT, NF-κB, and Rho/ROCK pathway networks, enabling a wide range of cellular responses including hypertrophy, proliferation, inflammatory cytokine expression, and extracellular matrix remodeling. These pleiotropic signaling systems make Human Angiotensin II especially valuable for modeling hypertension, endothelial dysfunction, oxidative stress, and fibrotic processes in controlled laboratory experiments. In addition, AT1R cross-talks with growth factor receptors, adrenergic receptors, and mechanosensitive channels, giving Angiotensin II a unique multipathway regulatory footprint useful for complex systems biology studies.
Human Angiotensin II can also bind the AT2 receptor (AT2R), although with different functional outcomes. AT2R generally opposes AT1R signaling by promoting vasodilation, nitric oxide (NO) production, anti-proliferative activity, and apoptosis. The balance between AT1R and AT2R responses enables researchers to design highly controlled experiments that dissect specific receptor contributions to vascular and renal physiology. This receptor duality makes Angiotensin II indispensable in multi-target pharmacology, receptor selectivity assays, and comparative peptide–receptor binding studies.
At the endocrine level, Human Angiotensin II exerts powerful effects on adrenal steroidogenesis by stimulating aldosterone secretion from the zona glomerulosa. This drives sodium retention and potassium excretion, thereby modulating blood volume and systemic blood pressure. These endocrine features make the peptide an important tool for studying salt-sensitive hypertension, RAAS overactivation, hormonal coupling with kidney function, and metabolic regulatory loops.
In neural systems, Angiotensin II modulates sympathetic output, thirst mechanisms, baroreflex sensitivity, and central inflammatory responses. These neuromodulatory actions support its use in neurovascular coupling research, stress-response modeling, and CNS–cardiovascular communication studies.
Because of its robust, predictable, and tiered signaling profile—from rapid calcium mobilization to long-term transcriptional regulation—Human Angiotensin II remains a foundational reagent in cardiovascular pharmacology, renal physiology, peptide receptor biology, and multi-omic systems research. Its mechanism of action is well-characterized, reproducible, and highly suitable for both reductionist and integrative biomedical research workflows.
Applications
Human Angiotensin II is also widely applied in computational modeling environments, where researchers integrate the peptide’s physicochemical parameters into cardiovascular simulation pipelines. These models help quantify receptor activation thresholds, peptide–enzyme turnover rates, and tissue‐specific diffusion coefficients. In large-scale systems biology maps, Angiotensin II frequently appears as a hub node connecting oxidative stress pathways, nitric oxide metabolism, and inflammatory cytokine cascades. Because of its defined sequence and stable experimental behavior, it is frequently used as a benchmark calibrator for validating peptide detection algorithms in LC–MS/MS proteomics workflows.
Furthermore, in microphysiological system development, Human Angiotensin II acts as a controllable vasoactive agent for testing microfluidic artery–on–chip platforms. Researchers use precise dose–response curves to assess chip sensitivity, endothelial barrier performance under flow, and the reproducibility of organ-on-chip readouts across batches. In pharmacodynamic screenings, Angiotensin II is employed to create stress-induced hypertensive microenvironments that support evaluation of anti-fibrotic, antioxidant, and anti-inflammatory drug candidates. These controlled hypertensive models are required for regulatory-grade validation studies, making the peptide valuable for CROs and academic–industry partnerships.
In renal physiology and nephrotoxicity research, Human Angiotensin II initiates glomerular hypertension, enabling observation of renal autoregulatory failure in cell lines, explants, and engineered 3D kidney tissues. It assists in evaluating renal endothelial dysfunction, altered transporter expression, and mesangial matrix expansion under pathological mechanical stress. Because these responses translate well across species, Angiotensin II is a core reagent for multi-species comparative renal research, supporting both advanced mechanistic studies and high-throughput compound screens.
Research Models
Human Angiotensin II is widely incorporated into multi-organ axis research models, particularly those investigating coordinated heart–kidney regulation. Combined cardiovascular–renal platforms demonstrate how Angiotensin II-driven vasoconstriction affects glomerular filtration, renal blood flow, and sodium handling in a synchronized system. These composite models help quantify systemic feedback loops, identify points of compensatory failure, and validate the efficacy of novel RAS-inhibiting therapies.
Another prominent category includes fibrosis progression models, where Angiotensin II acts as a potent inducer of fibroblast activation, collagen accumulation, and extracellular matrix remodeling. Researchers leverage this effect in liver, heart, lung, and kidney fibrosis systems. These models allow detailed profiling of pro-fibrotic signaling trajectories and serve as testing grounds for multi-target anti-fibrosis agents that operate at early or late stages of disease evolution.
Human Angiotensin II is also utilized in vascular mechanical stress models, particularly those involving biomechanical loading devices and 3D vascular scaffolds. By combining Angiotensin II with controlled pulsatile flow, researchers can replicate physiologic and pathological shear profiles to study endothelial mechanotransduction, cytoskeletal rearrangement, nitric oxide suppression, and oxidative stress amplification.
In neural injury models, Angiotensin II helps investigate neurovascular coupling changes, blood–brain barrier permeability, and microglial activation under hypertensive stress. These models support integrative research into vascular contributions to cognitive impairment, neuroinflammation, and tissue perfusion deficits.
Experimental Design Considerations
When designing experiments involving Human Angiotensin II, researchers should incorporate precise controls for environmental variables such as pH stability, calcium concentration, and dissolved gas levels, all of which significantly influence vascular contractility responses. Due to the peptide’s rapid action, real-time or high-frequency data acquisition (e.g., micropressure sensors, real-time impedance analysis) is strongly recommended to avoid missing transient physiological events.
Batch-to-batch consistency is essential. High-purity, GMP-like, or ISO-compliant manufacturing specifications minimize experimental variance. Researchers conducting long-term studies should consider aliquoting strategies to prevent freeze–thaw cycles that may reduce potency. For organ-on-chip or microtissue integration, flow-rate calibration ensures that Angiotensin II reaches intended microvascular beds at physiological concentrations without diffusion-related loss.
In cell-based assays, receptor density (AT1R and AT2R) strongly influences outcome variability. Verification using qPCR, Western blotting, or immunostaining strengthens reproducibility. Additionally, co-treatments involving oxidative stress modulators or RAS inhibitors must include carefully selected timepoints to avoid misinterpreting transient compensatory responses as primary effects.

Laboratory Safety & Handling Guidelines
Human Angiotensin II should be stored at –20°C or below, protected from repeated exposure to light and moisture. Researchers should handle all peptide powders and solutions using appropriate PPE, including gloves and protective eyewear, to avoid dermal or aerosol exposure. Avoid generating dust when opening vials. Use sterile, low-binding microtubes for preparation to prevent adsorption losses.
During solution preparation, dissolve the peptide gently using sterile water, PBS, or buffer recommended for your assay. Do not vortex aggressively, as this may degrade peptide integrity. If sterile filtration is required, use low-protein-binding filters to preserve concentration accuracy. Dispose of unused solutions and labware following institutional and local biosafety regulations, as peptide-based biological tools may require chemical or autoclave deactivation before disposal.
Integration with Multi-Omic & Computational Studies
The integration of Human Angiotensin II into multi-omic and computational research frameworks has become increasingly important for dissecting renin–angiotensin system (RAS) biology at systems-level resolution. In transcriptomic studies, Angiotensin II exposure enables researchers to map time-resolved gene expression changes across vascular, renal, and endocrine cells, revealing regulatory networks influencing inflammation, oxidative stress, and extracellular matrix remodeling. When combined with single-cell RNA sequencing (scRNA-seq), the peptide helps identify cell-type-specific receptor activation patterns, uncovering heterogeneity in AT1R- and AT2R-mediated signaling across complex tissues.
Proteomic and phospho-proteomic analyses further strengthen these findings by quantifying downstream signaling cascades, including MAPK, JAK/STAT, and calcium-dependent pathways activated by Human Angiotensin II. These datasets provide a molecular atlas of receptor-linked phosphorylation events and post-translational modifications. Metabolomics and lipidomics add complementary insights by tracking metabolic shifts associated with vasoconstriction, oxidative stress, and cardiovascular remodeling, creating a holistic biochemical profile of Angiotensin II-driven physiological responses.
Computational modeling and systems pharmacology approaches allow prediction of receptor kinetics, intracellular pathway activation, dose–response relationships, and network perturbations. Machine learning–enhanced simulations can integrate omics datasets to identify biomarkers of peptide response or detect synergistic interactions with co-administered stimuli such as cytokines, adrenergic agonists, or oxidative stressors. In vascular research, in silico hemodynamic modeling enables simulation of Angiotensin II–induced changes in vascular tone and flow resistance.
Overall, multi-omic integration greatly enhances the resolution at which researchers can study Human Angiotensin II, helping bridge molecular mechanisms with tissue-level and systemic physiological outcomes. This systems-biology approach supports biomarker discovery, drug-target validation, and mechanistic modeling across cardiovascular, renal, inflammatory, and metabolic research fields.
Side Effects (Research Observations Only)
In controlled laboratory studies, Human Angiotensin II has demonstrated a range of concentration-dependent physiological responses that researchers should account for when designing experiments. At higher experimental doses, notable observations include enhanced vasoconstriction, measurable increases in perfusion pressure, and rapid fluctuations in vascular resistance across isolated tissue preparations. These hemodynamic responses are expected based on the peptide’s potent activation of AT1 receptors, but researchers should still document these effects carefully to ensure reproducibility.
Cell-based and tissue-based models have reported elevated oxidative stress markers, including increased ROS generation and upregulation of pro-inflammatory cytokine transcripts. These responses may contribute to downstream remodeling processes such as fibroblast activation, hypertrophic signaling, or extracellular matrix deposition. In multi-organ systems, Human Angiotensin II exposure has occasionally been associated with altered renal filtration metrics, shifts in electrolyte transport pathways, and changes in endothelial barrier permeability—findings that mirror its biological roles but may influence experimental outcomes.
While these observations are typical for Angiotensin II research, they underscore the importance of using appropriate controls, exposure windows, and analytical endpoints. Importantly, these research-only side effects do not imply suitability for diagnostic, therapeutic, or physiological manipulation in humans or animals. All findings should be interpreted solely within the context of laboratory experimentation.
Keywords
Human Angiotensin II; Ang II Peptide; High-Purity Angiotensin II; AT1 Receptor Agonist; Vasoactive Research Peptide; Cardiovascular Signaling Peptide; Renin–Angiotensin System Modulator; Laboratory-Grade Peptide; Custom Peptide Manufacturing; GMP Peptide Supplier; Bulk Angiotensin II Powder; Peptide Hormone Research; Endothelial Function Studies; Vascular Biology Reagent; Low-Cost Peptide Wholesale Supply.
Shipping Guarantee
All shipments of Human Angiotensin II are handled under strict temperature-controlled conditions (2–8 °C) to preserve peptide integrity and bioactivity. Moisture-protected packaging and sterile, tamper-evident containers ensure the material remains stable throughout transit. Each order includes global tracking, batch documentation, and quality verification to give researchers full confidence in product consistency and delivery reliability.

Trade Assurance
We provide factory-direct manufacturing with high-purity, GMP-compliant production of Human Angiotensin II, ensuring consistent quality across all batches. Bulk quantities, OEM customization, and peptide modification services are available to support specialized research demands. Each shipment includes COA, MSDS, HPLC/UV data, and full QC traceability, ensuring transparency and dependable quality assurance for institutional and commercial laboratories.
Payment Support
We offer multiple secure international payment options, including bank transfer, corporate credit, PayPal, major credit cards, and cryptocurrency for eligible customers. Bulk, multi-unit, and OEM orders can benefit from flexible payment arrangements tailored to research institutions. All transactions are fully documented to ensure transparency, traceability, and compliance. Our systems are designed for efficient, reliable, and global procurement of high-purity research peptides.
Disclaimer
Human Angiotensin II is provided strictly for laboratory research use only. It is not intended for human or veterinary use, medical procedures, diagnostic applications, or consumption. Researchers must follow institutional biosafety rules, chemical handling standards, and peptide-specific regulatory guidelines when working with this product. All experimental use is the responsibility of the purchasing laboratory.
Additional information
| Weight | 0.5 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |
1. What is Human Angiotensin II used for in research?
Human Angiotensin II is widely used to study cardiovascular regulation, vasoconstriction mechanisms, and renin–angiotensin system (RAS) signaling. Researchers employ it to model hypertension, vascular remodeling, and endothelial dysfunction in both in vitro and in vivo systems. Its high reproducibility and strong receptor-specific activation make it ideal for mechanistic and drug-screening studies.
2. Is Human Angiotensin II suitable for in vivo studies?
Yes, Human Angiotensin II is commonly applied in animal models to induce predictable cardiovascular responses, such as elevated blood pressure or altered renal hemodynamics. The peptide is highly potent, so precise dosage calibration is required for safe and reproducible experimental outcomes. Researchers should also ensure proper sterile preparation and delivery via validated routes.
3. What purity level is recommended for signal-transduction research?
For receptor-level and pathway-specific studies, ≥98% purity is strongly recommended to minimize off-target artifacts. High-purity Human Angiotensin II ensures that observed biological effects originate from AT1/AT2 receptor engagement rather than peptide impurities. This level of purity is especially critical in quantitative, multi-omic, or computationally assisted modeling research.
4. How should Human Angiotensin II be reconstituted?
Reconstitution should be performed using sterile, peptide-grade solvents such as water or saline. Once dissolved, the solution must be handled under aseptic conditions and aliquoted to avoid repeated freeze–thaw cycles. Researchers often prepare working concentrations immediately before experiments to ensure maximal stability and biological activity.
5. What storage conditions maintain peptide integrity?
The lyophilized peptide should be stored at 2–8 °C to preserve long-term stability and minimize degradation. After reconstitution, aliquots should be kept frozen and protected from light exposure. Avoiding moisture and repeated thawing is essential for maintaining consistent potency across experiments.
6. Do batch-to-batch variations affect study reproducibility?
High-quality, GMP-grade Human Angiotensin II minimizes batch variation through standardized synthesis, purification, and analytical QC. Each lot is verified with HPLC, MS, and purity profiling to ensure consistent performance. Researchers can expect highly reproducible experimental outputs across different batches when proper handling is applied.
7. Can Human Angiotensin II be used with high-throughput screening platforms?
Yes, the peptide’s predictable AT1 receptor activation makes it compatible with high-throughput assays. Researchers frequently integrate it into automated screening workflows to characterize pathway modulators or test small-molecule antagonists. Its stability and reliability allow seamless incorporation into robotics-based systems and multi-well assay formats.
8. Is Human Angiotensin II compatible with omics-based research?
Absolutely. Human Angiotensin II is often used to stimulate pathway-specific responses measured through transcriptomics, proteomics, metabolomics, and phosphoproteomics. This enables deep mechanistic insight into vascular biology, inflammation, and stress responses. Its defined mechanism of action supports high-confidence computational modeling and network analysis.
9. Are solvent carriers important when preparing working solutions?
Yes, the choice of solvent influences stability and biological performance. Researchers generally use sterile water or saline, avoiding solvents that may alter peptide structure or receptor affinity. Carrier selection should align with downstream applications, especially in sensitive cellular assays.
10. Can custom modifications of Human Angiotensin II be manufactured?
Custom analogs, isotopic labels, and sequence modifications can be produced for specialized research applications. These modifications may enhance stability, receptor-selectivity, or analytical detectability in complex biological samples. OEM peptide customization ensures tailored solutions for advanced pharmacology, receptor-binding, or multi-omic projects.
















Reviews
There are no reviews yet.