No products in the cart.
Sale
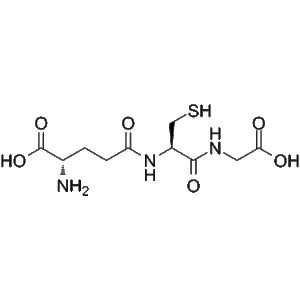
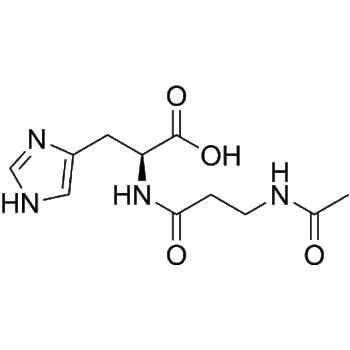
L-Glutathione reduced | CAS 70-18-8 | Endogenous Antioxidant for Skin Brightening and Anti-Aging Research
Original price was: $32.00.$23.00Current price is: $23.00.
L-Glutathione reduced (GSH; γ-L-Glutamyl-L-cysteinyl-glycine) is a tripeptide antioxidant widely used in cosmetic and dermatological research. It supports skin-brightening, anti-aging, and cellular protection studies through its redox-regulating and detoxifying properties.
Description
Product Description
L-Glutathione reduced (GSH; γ-L-Glutamyl-L-cysteinyl-glycine) is a naturally occurring tripeptide composed of glutamate, cysteine, and glycine. It functions as one of the most potent intracellular antioxidants, maintaining cellular redox equilibrium and protecting against oxidative damage caused by reactive oxygen species (ROS). Within the realm of cosmetic peptide research, L-Glutathione reduced has attracted considerable attention due to its remarkable ability to reduce melanin synthesis, improve skin brightness, and support anti-aging mechanisms at the cellular level.
In skin-related studies, L-Glutathione reduced plays a multifaceted biochemical role. Its thiol (-SH) group acts as a reducing agent that neutralizes hydrogen peroxide, lipid peroxides, and hydroxyl radicals. Through the enzymatic cycling between its reduced (GSH) and oxidized (GSSG) forms, this peptide sustains the antioxidant defense network involving glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST). The continuous regeneration of GSH ensures redox stability in epidermal and dermal cells, mitigating photoaging and oxidative stress induced by UV exposure and environmental pollutants.
From a cosmetic research perspective, L-Glutathione reduced is widely studied for its skin-brightening effects. It has been shown to inhibit melanogenesis by modulating the activity of tyrosinase and shifting eumelanin synthesis toward pheomelanin production, resulting in lighter and more even-toned skin. In vitro studies on melanocyte cultures demonstrate that GSH downregulates tyrosinase transcription and decreases melanin accumulation, suggesting a molecular mechanism behind its skin-whitening action. Moreover, GSH also contributes to the detoxification of quinones and peroxides produced during melanogenesis, further supporting its anti-pigmentation potential.
The peptide’s anti-aging applications in cosmetic research stem from its role in counteracting oxidative damage to lipids, proteins, and DNA. Chronic oxidative stress accelerates cellular senescence, collagen degradation, and dermal matrix weakening—all key factors in skin aging. L-Glutathione reduced serves as a protective molecule by restoring intracellular antioxidant capacity and supporting enzymatic systems like superoxide dismutase (SOD) and catalase. In fibroblast and keratinocyte models, GSH supplementation correlates with enhanced collagen synthesis and improved mitochondrial function, key indicators of cellular vitality and resilience.
In addition to antioxidant and brightening effects, L-Glutathione reduced is involved in detoxification and cellular metabolism. Acting as a cofactor for detoxifying enzymes, it conjugates with xenobiotic compounds and electrophiles, facilitating their elimination from cells. This property makes GSH particularly relevant in cosmetic formulations designed to study pollution-defense or anti-toxic skin protection. Furthermore, GSH influences cellular signaling by modulating transcription factors such as NF-κB and Nrf2, thereby regulating the expression of antioxidant response elements (ARE) that promote the synthesis of detoxification enzymes.
In research involving oxidative stress, L-Glutathione reduced is often explored in combination with other cosmetic peptides and cofactors like vitamin C, α-lipoic acid, and coenzyme Q10. These synergistic systems simulate the skin’s natural defense mechanisms and provide a model for studying the integrated antioxidative response. The reduced form of glutathione, as opposed to its oxidized counterpart, directly participates in electron transfer reactions that restore ascorbate and tocopherol (vitamin E) levels, forming a comprehensive antioxidant network.
As a peptide widely present in skin and other tissues, L-Glutathione reduced provides a valuable research tool for elucidating mechanisms of oxidative protection, detoxification, and melanogenesis regulation. It helps researchers investigate how redox balance affects cell differentiation, barrier integrity, and regenerative capacity. These properties underscore its importance in modern cosmetic science, where understanding intracellular defense pathways is key to developing next-generation skincare research formulations.

Product Specifications
| Item | Description |
|---|---|
| Product Name | L-Glutathione reduced |
| CAS Number | 70-18-8 |
| Synonyms | GSH; γ-L-Glutamyl-L-cysteinyl-glycine; Reduced glutathione |
| Molecular Formula | C10H17N3O6S |
| Molecular Weight | 307.32 g/mol |
| Purity | ≥99% |
| Appearance | White to off-white crystalline powder |
| Solubility | Soluble in water |
| Storage | Store at −20°C, protected from light and moisture |
| Category | Cosmetic peptide; Antioxidant peptide |
| Applications | Skin-brightening research, anti-aging studies, oxidative stress modeling, melanin inhibition |
| Research Area | Dermatology, antioxidant biology, redox regulation, cosmetic science |
| Supplier Type | Research use only reagent |
| Intended Use | For laboratory research use only |
Mechanism of Action
L-Glutathione reduced operates primarily through its thiol group, which donates hydrogen atoms to neutralize reactive oxygen species (ROS). In the skin, this activity reduces oxidative damage to cellular components and prevents peroxidation of membrane lipids. It is part of the glutathione redox couple (GSH/GSSG), a central regulator of intracellular redox potential. The continuous interconversion between these two forms, catalyzed by glutathione reductase, enables cells to maintain antioxidant capacity even under stress.
In melanogenesis, GSH exerts control by interacting with melanogenic intermediates. It forms conjugates with dopaquinone, diverting it from the eumelanin synthesis pathway toward the formation of lighter-colored pheomelanin. Additionally, GSH modulates the expression of tyrosinase-related proteins, suppressing excessive pigmentation and promoting even skin tone. These molecular mechanisms make it a prominent focus in cosmetic whitening and anti-pigmentation research.
GSH also acts as a cellular protector by supporting enzymatic detoxification. It conjugates with harmful electrophiles via the catalytic activity of glutathione-S-transferase (GST), facilitating their export from cells. This process plays a vital role in maintaining healthy skin barrier functions and in protecting keratinocytes and fibroblasts from chemical-induced oxidative damage.
At the signaling level, GSH influences transcription factors such as Nrf2, a master regulator of antioxidant defense genes. By maintaining Nrf2 in its active state, GSH promotes the upregulation of detoxifying and protective enzymes. This cascade supports the repair of oxidative lesions and the synthesis of new cellular components, which is particularly relevant in studies of skin rejuvenation and tissue regeneration.
Side Effects
In laboratory research conditions, L-Glutathione reduced demonstrates an excellent safety profile. It is a naturally occurring intracellular molecule and typically well tolerated by cultured cells. However, at very high concentrations, excessive reducing power may alter cellular redox balance, leading to potential interference with experimental oxidative stress induction models. Researchers should calibrate doses carefully based on cell type and assay objectives.
As a cosmetic peptide research compound, L-Glutathione reduced should not be applied to human or animal subjects. It is intended strictly for in vitro and ex vivo research. Proper laboratory handling procedures and personal protective equipment are recommended during experimentation.
Keywords
L-Glutathione reduced, CAS 70-18-8, GSH peptide, skin brightening, antioxidant peptide, anti-aging research, melanin inhibition, redox regulation, glutathione cycle, cosmetic peptide
Shipping Guarantee
All shipments are handled using validated cold-chain logistics to preserve peptide integrity. Each package is sealed in moisture-proof containers with secondary protective wrapping and continuous temperature monitoring. Products are shipped via express international couriers with full tracking and insurance coverage.
Trade Assurance
We ensure product authenticity, verified ≥99% purity, and compliance with analytical standards (HPLC, MS, and NMR). Each batch is supplied with a Certificate of Analysis (CoA). Our trade assurance policy guarantees replacement or refund for any deviation from listed specifications.
Payment Support
We provide flexible and secure global payment options to support international research transactions. Accepted payment methods include PayPal, major credit cards (Visa, MasterCard, American Express), telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are protected by industry-standard encryption and verified payment gateways to ensure confidentiality and fund security.
Disclaimer
All products listed are intended for laboratory research use only and not for human or veterinary use. They are not drugs, medical devices, or diagnostics and should not be administered to humans or animals. Researchers must handle all materials in accordance with institutional biosafety and chemical safety guidelines. The information provided is for scientific reference only and does not imply therapeutic efficacy, safety, or regulatory approval.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 65 × 53 × 65 cm |
What is the primary research use of L-Glutathione reduced?
It is used for oxidative stress, skin brightening, and anti-aging research in cosmetic and biochemical studies.
Is L-Glutathione reduced suitable for human application?
No. It is strictly for laboratory research use only, not for cosmetic or therapeutic application.
How should it be stored?
Store under −20°C, protected from light and moisture, to maintain peptide stability.
What is its purity level?
Each batch is verified at ≥99% purity by HPLC and MS analysis.
Can it be used in combination with vitamin C in research?
Yes, it is often studied with vitamin C for synergistic antioxidant effects.
Does it affect melanin synthesis?
Yes, L-Glutathione reduced inhibits tyrosinase and promotes pheomelanin production in melanocytes.
Is it water-soluble?
Yes, L-Glutathione reduced is freely soluble in water.
Does it protect against photoaging?
Research indicates it helps mitigate oxidative stress induced by UV exposure.
What are the key assays used for GSH research?
Common assays include redox potential measurement, ROS quantification, and tyrosinase activity inhibition tests.
Is analytical data provided with the product?
Yes. Each lot comes with a full Certificate of Analysis (CoA) confirming identity and purity.














Reviews
There are no reviews yet.