No products in the cart.
Sale
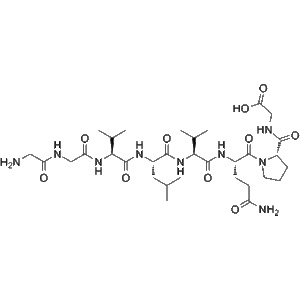
Larazotide (CAS 258818-34-7) | Orally Active Zonulin Antagonist for Celiac Disease & Antiviral Research
Original price was: $23.00.$17.00Current price is: $17.00.
Larazotide is a synthetic peptide that functions as a zonulin antagonist, regulating tight junctions in the intestinal epithelium. Larazotide exhibits antiviral activity against varicella-zoster virus (VZV) and is a powerful tool for celiac disease and infection research. Available from a GMP-certified supplier for wholesale and retail laboratory use.
Description
Product Description
Larazotide (CAS 258818-34-7) is a synthetic peptide that serves as a potent and orally active zonulin antagonist. Zonulin is a protein that regulates the permeability of tight junctions in the intestinal epithelium, playing a critical role in intestinal barrier function. Dysregulation of zonulin has been associated with gastrointestinal disorders such as celiac disease, inflammatory bowel disease, and other autoimmune conditions.
Larazotide has been shown to modulate tight junctions, restoring barrier integrity and reducing intestinal hyperpermeability. Its mechanism allows for oral administration, making it a convenient research tool for in vivo and in vitro studies focused on intestinal epithelium function.
Beyond its role in intestinal health, Larazotide exhibits antiviral activity against varicella-zoster virus (VZV). Laboratory studies demonstrate EC50 values of 44.14 ?M for VZV strain OKA and 59.06 ?M for strain 07-1, highlighting its potential in infection research. This dual function—intestinal barrier modulation and antiviral activity—makes Larazotide a versatile peptide for experimental studies across gastroenterology, virology, and immunology.
In preclinical studies, Larazotide has been used to:
Investigate celiac disease mechanisms, including gliadin-induced zonulin release.
Assess tight junction integrity and intestinal permeability in various cell models.
Study viral entry and replication of VZV in epithelial cells.
Explore immune system interactions associated with barrier dysfunction and viral infection.
Supplied as a lyophilized peptide powder, Larazotide is stable under appropriate storage conditions and produced in a GMP-certified facility, ensuring high purity and reproducibility for research applications.
For laboratory research use only. Not for human or veterinary use.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Larazotide |
| Synonyms | Larazotide acetate, AT-1001 |
| CAS Number | 258818-34-7 |
| Molecular Type | Peptide |
| Purity | ? 98% (HPLC) |
| Appearance | Lyophilized peptide powder |
| Mechanism | Zonulin antagonist, tight junction modulator, antiviral activity |
| EC50 | 44.14 ?M (VZV strain OKA), 59.06 ?M (VZV strain 07-1) |
| Stability | Stable at -20°C; avoid repeated freeze-thaw cycles |
| Storage Conditions | Store at -20°C under inert atmosphere |
| GMP Compliance | Produced in GMP-certified facility |
| Application | Laboratory research in celiac disease, intestinal barrier function, viral infections |
| Availability | Wholesale & retail supply |
Mechanism of Action & Research Applications
Larazotide’s primary mechanism is inhibition of zonulin, a physiological modulator of tight junctions between epithelial cells. By blocking zonulin signaling, Larazotide prevents tight junction disassembly, maintaining intestinal barrier integrity. This property is particularly valuable in research on celiac disease, where gliadin exposure triggers zonulin release, leading to increased intestinal permeability, immune activation, and inflammation.
Key Mechanisms:
Zonulin Antagonism:
Binds to zonulin receptors, blocking their action on tight junction proteins (e.g., occludin and claudins).
Prevents intestinal hyperpermeability associated with autoimmune and inflammatory responses.
Antiviral Activity:
Exhibits selective activity against varicella-zoster virus (VZV).
Interferes with viral entry or replication in epithelial cells.
Oral Bioactivity:
Stable and active when administered orally, enabling in vivo studies without injection.
Suitable for animal models and cell-based assays.
Research Applications:
Celiac Disease Research:
Study gliadin-induced intestinal barrier dysfunction.
Evaluate potential therapeutic interventions targeting tight junction regulation.
Intestinal Barrier Function:
Investigate tight junction protein expression and localization.
Examine effects on epithelial permeability under stress or inflammatory conditions.
Antiviral Studies:
Test activity against VZV and other viruses in epithelial and immune cell models.
Analyze interactions between viral infection and intestinal permeability.
Immunological Research:
Study the link between intestinal barrier dysfunction and immune activation.
Explore cytokine signaling and inflammation modulation by Larazotide.
Combination Therapy Screening:
Evaluate synergistic effects with probiotics, antivirals, or anti-inflammatory agents.
Larazotide is therefore a versatile research tool for both gastroenterology and virology labs, enabling mechanistic studies, preclinical evaluations, and the development of barrier-protective strategies.
Side Effects (For Reference in Research Models)
In preclinical studies, Larazotide shows minimal cytotoxicity in cell culture models. Observed effects in laboratory settings include:
Restoration of tight junction integrity in epithelial cell monolayers.
Reduced intestinal permeability under gliadin or stress-induced conditions.
Selective antiviral activity against VZV, without broad cytotoxicity.
Peptide stability considerations: Protect from repeated freeze-thaw cycles to maintain activity.
Strictly for laboratory research use. Not for human or veterinary applications.
Disclaimer
Larazotide is intended solely for research purposes. It is not approved for clinical, human, or veterinary use. Researchers should adhere to laboratory safety protocols when handling the peptide.
Keywords
Larazotide
AT-1001 peptide
Zonulin antagonist peptide
Oral tight junction modulator
Celiac disease research peptide
Intestinal barrier research
Varicella-zoster virus antiviral peptide
Laboratory use only peptide
Additional information
| Weight | 0.7 kg |
|---|---|
| Dimensions | 54 × 28 × 54 cm |














Reviews
There are no reviews yet.