No products in the cart.
Sale
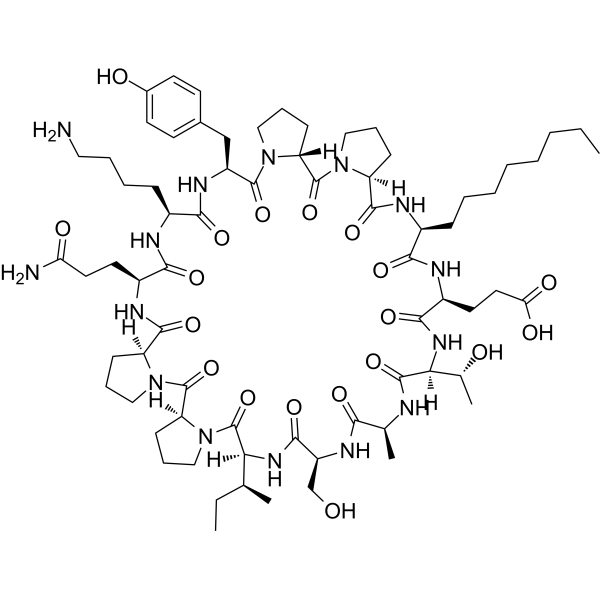
Lonodelestat (POL6014) – Potent Peptide Inhibitor of Human Neutrophil Elastase – GMP Peptide
Original price was: $16.00.$12.00Current price is: $12.00.
Lonodelestat (POL6014) is a synthetic, GMP-grade peptide that potently and selectively inhibits human neutrophil elastase (hNE). With a purity of ~98–99%, it supports inhalation-based research in cystic fibrosis, ARDS, and neutrophilic lung diseases. Available for wholesale and retail. For research use only.?If you need to place an order or inquire about wholesale product prices, specifications and uses, please contact our staff?
Description
Lonodelestat (also known as POL6014; CAS No. 906547-89-5) is a highly potent, orally active, and selective peptide inhibitor of human neutrophil elastase (hNE). This peptide is engineered to target hNE, a protease released by activated neutrophils that contributes to lung tissue degradation, chronic inflammation, and disease progression in cystic fibrosis (CF) and other neutrophil-driven pulmonary conditions.
Developed for inhalation-based delivery, Lonodelestat achieves high concentrations in the lung while maintaining low systemic exposure—ideal for precision regulation of hNE in research models. Purity levels range from 98–99%, certified by GMP-grade synthesis, enabling reproducible and reliable results in experimental settings.
Lonodelestat is currently under clinical investigation for CF, with Phase 1a and 1b studies demonstrating tolerability, dose-linear pharmacokinetics, and robust hNE inhibition in sputum following inhalation. These data underline its potential as a therapeutic model compound for studying neutrophil-mediated lung injury, inflammatory pathways, and pulmonary disease mechanisms.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Lonodelestat (POL6014) |
| CAS Number | 906547-89-5 |
| Synonyms | POL6014, Lonodelestat |
| Chemical Formula | C??H???N??O?? |
| Molecular Weight | ? 1478.73 g/mol |
| Purity | ~98–99% (GMP-grade) |
| Form | Lyophilized powder |
| Appearance | White to off-white solid |
| Storage Conditions | Store at –80 °C (powder); –20 °C short-term |
| Solubility | Soluble in water or DMSO |
| Applications | Cystic fibrosis, neutrophil elastase inhibition, lung inflammation models |
| Manufacturer | GMP-certified supplier |
Mechanism of Action & Research Applications
Mechanism of Action
Lonodelestat functions as a reversible and selective inhibitor of human neutrophil elastase (hNE), a key enzyme responsible for elastin and extracellular matrix degradation in inflamed lung tissue. Administered via inhalation, it achieves high pulmonary concentrations while minimizing systemic exposure, effectively suppressing hNE activity in the lung’s epithelial lining fluid.
Research Applications
Cystic Fibrosis (CF) models: Lonodelestat is used to study control of hNE-mediated lung damage and inflammation progression, with effectiveness demonstrated in Phase 1 studies.
Acute Respiratory Distress Syndrome (ARDS) and neutrophilic lung diseases: Preclinical findings support its use in ARDS models to inhibit hNE-driven inflammatory cascades and NET formation.
Chronic pulmonary conditions such as COPD, non-CF bronchiectasis, AAT deficiency, and acute lung injury (ALI): Lonodelestat shows promise as a candidate to mitigate progressive tissue damage.
Pharmacokinetic (PK) & Pharmacodynamic (PD) studies: Its inhaled delivery and linear dose-response enable nuanced modeling of pulmonary drug exposure, retention, and functional outcomes.
Development & Formulation Notes
Lonodelestat was developed using peptide engineering approaches to ensure high selectivity, reversibility, and pulmonary retention. The inhaled formulation enables localized action with minimal systemic absorption—a design that benefits safety and translational modeling.
Manufactured under GMP standards, Lonodelestat is provided as a lyophilized powder to ensure stability and ease of reconstitution. Packaging is optimized for both small-scale laboratory studies and larger institutional usage. Each lot is accompanied by a Certificate of Analysis (CoA) confirming purity, identity, and appropriate storage parameters.
Researchers investigating therapeutic approaches to lung inflammation, elastase inhibition, and peptide drug design will find Lonodelestat a robust and validated investigational tool.

Side Effects (For Research Reference Only)
In preclinical and early-phase studies, Lonodelestat has demonstrated:
Excellent tolerability up to 160 mg inhaled doses in CF patients and healthy volunteers.
No serious adverse events or grade 3+ events reported during single- and multiple-dose administration.
Further models may explore effects on airway reactivity, inflammation markers, and long-term pulmonary function under sustained dosing protocols.
Disclaimer
Lonodelestat (POL6014) is strictly for laboratory research use only. It is not approved for human or veterinary use, diagnostics, or therapeutic applications. All usage should comply with institutional regulations and safety protocols.
Logistics & Packaging
Packaging: Secure, sterile vials containing lyophilized Lonodelestat.
Shipping: Temperature-controlled shipping recommended to preserve compound integrity.
Storage: Store at –80 °C for long-term; dedicated aliquots minimize degradation from freeze-thaw.
Supply Options: Wholesale and retail quantities available, with flexible batch sizes to meet diverse laboratory demands.
Keywords
Lonodelestat; POL6014; CAS No. 906547-89-5; human neutrophil elastase inhibitor; hNE inhibitor; cystic fibrosis research peptide; GMP-grade elastase inhibitor; inhaled elastase inhibitor; pulmonary inflammation research; long-acting peptide inhibitor; research-use-only peptide.
Additional information
| Weight | 0.8 kg |
|---|---|
| Dimensions | 86 × 56 × 86 cm |













Reviews
There are no reviews yet.