No products in the cart.
Lonodelestat TFA – GMP Manufacturer
$2.00
Lonodelestat TFA is a GMP-grade, high-purity (99.86%) peptide inhibitor of human neutrophil elastase (hNE), orally active and selective. Ideal for research in cystic fibrosis (CF), acute lung injury, and inflammatory lung diseases. For laboratory research use only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
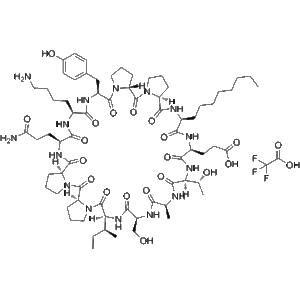
Lonodelestat TFA (also known as POL6014 TFA) is a potent, orally active, and highly selective peptide inhibitor of human neutrophil elastase (hNE). Preclinical and early clinical data support its use in cystic fibrosis (CF) research and other pulmonary inflammatory conditions. In Phase 1 trials, inhaled Lonodelestat demonstrated high lung concentrations, effective hNE inhibition, and good tolerability in both healthy volunteers and CF subjects. Manufactured under GMP standards with a purity of 99.86%, this compound offers reliable performance for laboratory research applications. Not for clinical or therapeutic use.
Product Specifications
| Parameter | Details |
|---|---|
| Product Name | Lonodelestat TFA |
| Synonyms | POL6014 TFA; Lonodelestat |
| Purity | 99.86% (GMP-grade) |
| Mechanism | Orally active, selective peptide inhibitor of human neutrophil elastase (hNE) |
| CAS No. | (Not specified in sources) – consider confirming internally |
| Molecular Formula / Weight | (Not publicly detailed) – refer to manufacturer data sheet |
| Appearance | White to off-white powder (typical peptide appearance) |
| Solubility | Likely soluble in DMSO or aqueous buffers (peptide properties) |
| Storage Conditions | Store at –20 °C; protect from light and moisture |
| Manufacturing Standard | GMP-compliant |
| Applications | Cystic fibrosis research, acute lung injury models, pulmonary inflammation studies |
| Regulatory Status | For laboratory research use only |
Mechanism of Action & Research Applications
Lonodelestat TFA acts as a highly potent and selective inhibitor of human neutrophil elastase (hNE), an enzyme implicated in lung tissue damage, mucus hypersecretion, and inflammatory response—especially in diseases like cystic fibrosis (CF).
Key Research Applications:
Cystic Fibrosis (CF): Inhalation of Lonodelestat achieves high pulmonary concentrations with near-complete suppression of hNE in sputum, supporting studies of lung inflammation mitigation.
Acute Lung Injury (ALI) & ARDS: Demonstrated efficacy in animal models of neutrophil-mediated lung injury and inflammation.
Pulmonary Hypertension (PAH): Preclinical models show reductions in right ventricular hypertrophy and improved vascular parameters.
Inflammatory Lung Disease Research: Useful across multiple pulmonary pathologies involving neutrophilic inflammation.

Side Effects (For Reference Only in Models)
Phase 1 studies indicated good tolerability and safety, with no serious adverse events reported. Transient effects may include mild respiratory discomfort or local reactions. Not to be used in clinical practice.
Disclaimer
Lonodelestat TFA is strictly intended for laboratory research use only. NOT approved for clinical, therapeutic, or human use. Follow institutional guidelines and safety protocols when handling.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 26 × 23 × 26 cm |














Reviews
There are no reviews yet.