No products in the cart.
Lparomlimab & Tuvonralimab Injection
$1.00
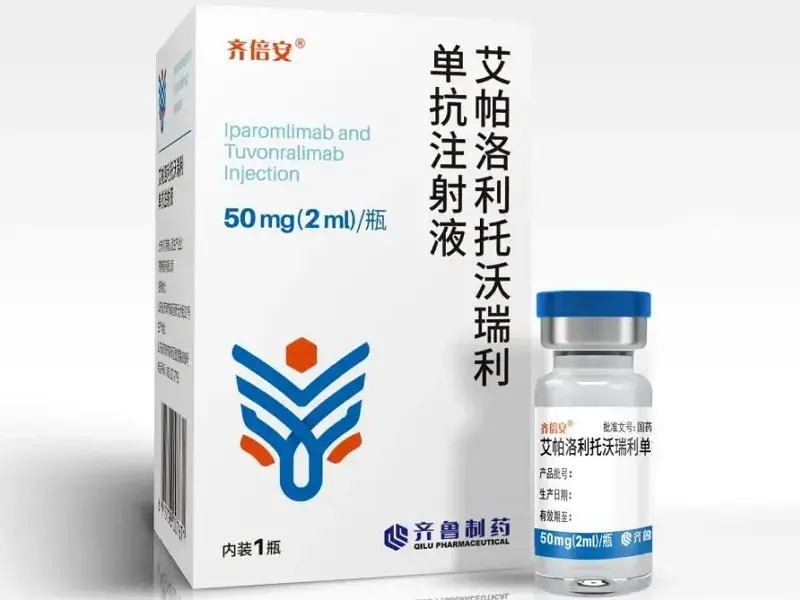
Lparomlimab & Tuvonralimab Injection Qibeian is a bi-functional monoclonal antibody combination developed by Qilu Pharmaceutical (QL1706), supplied as 50mg in 2mL vials. Approved by China’s NMPA (conditional marketing authorization No. S20240044; drug code 86904021005238). Intended exclusively for lab and investigative research applications, not for clinical or human use.
Description
Lparomlimab & Tuvonralimab Injection Product Specifications
| Attribute | Information |
|---|---|
| Product Name | Lparomlimab & Tuvonralimab Injection (Qibeian® / QL1706) |
| Formulation | Injection, 50mg in 2?mL vial |
| Approval No. | China NMPA S20240044 |
| Drug Standard Code | 86904021005238 |
| Manufacturer | Qilu Pharmaceutical Co., Ltd. |
| Intended Use | For research use only, not for therapeutic use |
Lparomlimab & Tuvonralimab Background & Mechanism
Qibeian (QL1706) combines two antibodies—lparomlimab (anti-PD?1) and tuvonralimab (anti-CTLA 4)—in a single product. This dual blockade enhances T-cell activation through simultaneous PD 1/CTLA 4 pathway inhibition
Conditionally approved by NMPA in September 2024 for recurrent/metastatic cervical cancer post-platinum therapy
Lparomlimab & Tuvonralimab Clinical Highlights
Phase II (DUBHE C 206): In recurrent/metastatic cervical cancer, objective response rate (ORR): 33.8%, disease control rate (DCR): 64.9%, median PFS: 5.4 months (148 patients)
Safety: 70.3% experienced treatment?related adverse events; 24.3% had grade ?3 TRAEs—most commonly anemia, thyroid dysfunction, GGT elevation
Research Applications
Ideal for preclinical and translational studies on:
Immune checkpoint inhibition
Tumor microenvironment research
Combination studies (e.g., with chemotherapy, targeted agents, radiotherapy)
Biomarker and pharmacodynamic assays
Storage & Handling
Storage: 2–8°C, avoid freezing.
Handling: Use aseptic technique in a biosafety cabinet.
Stability: Per manufacturer instructions (CCF refrigerated vials).
References
NMPA conditional approval announcement pubmed.ncbi.nlm.nih.gov+4english.nmpa.gov.cn+4english.nmpa.gov.cn+4
First approval review in Drugs journal biospace.com+15pubmed.ncbi.nlm.nih.gov+15bydrug.pharmcube.com+15
DUBHE C 206 Phase II trial data
Preclinical combination studies
Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 18 × 16 cm |












Reviews
There are no reviews yet.