No products in the cart.
Sale
Nilotinib Capsules | CAS 641571-10-0 | High-Purity BCR-ABL Tyrosine Kinase Inhibitor
Original price was: $5.00.$2.00Current price is: $2.00.
Nilotinib Capsules (CAS 641571-10-0) are high-purity BCR-ABL tyrosine kinase inhibitors in capsule form, designed for preclinical leukemia research. They target BCR-ABL kinase, enabling studies on cellular proliferation, apoptosis, and drug resistance mechanisms in hematologic malignancy models.
Description
Product Description
Nilotinib Capsules (CAS 641571-10-0) are a high-purity formulation of a selective BCR-ABL tyrosine kinase inhibitor, widely used in preclinical leukemia research. Nilotinib selectively inhibits BCR-ABL fusion proteins, including mutated forms resistant to first-generation inhibitors, providing a robust tool for mechanistic studies of chronic myeloid leukemia (CML) and related hematologic malignancies.
Mechanism and Molecular Background
The BCR-ABL fusion protein, resulting from the Philadelphia chromosome translocation, drives constitutive tyrosine kinase activity, promoting uncontrolled proliferation and survival of leukemic cells. Nilotinib binds to the ATP-binding site of BCR-ABL, inhibiting its phosphorylation activity, and downstream signaling pathways such as MAPK, PI3K/Akt, and STAT5, ultimately inducing apoptosis and cell cycle arrest in leukemic cells.
The capsule formulation allows reproducible dosing in in vitro and in vivo preclinical models, maintaining high stability and consistent bioavailability. Researchers can model dose-dependent effects on cellular proliferation, apoptosis induction, and signaling modulation using Nilotinib Capsules.
Preclinical Research Applications
Nilotinib Capsules are used for multiple experimental setups:
Cellular Proliferation Assays: Quantifying growth inhibition in BCR-ABL-positive leukemia cell lines.
Apoptosis Studies: Assessing caspase activation, Annexin V staining, and DNA fragmentation following BCR-ABL inhibition.
Drug Resistance Mechanism Studies: Investigating mutations in BCR-ABL that confer resistance to first-generation tyrosine kinase inhibitors.
Signal Transduction Analysis: Evaluating downstream effects on MAPK/ERK, PI3K/Akt, and STAT pathways.
Combination Therapy Research: Testing synergistic effects with chemotherapeutic agents or targeted small molecules in preclinical leukemia models.
The high-purity formulation (>99%) ensures that experimental effects are attributable to BCR-ABL inhibition, minimizing variability due to impurities or batch differences.
Quality and Stability
Nilotinib Capsules are manufactured under GMP-compliant conditions, ensuring reproducibility and functional consistency. Each batch is tested using HPLC, MS, and NMR for purity, identity, and structural integrity. Capsules are stable under ambient and refrigerated conditions, allowing flexible storage and experimental use.
The reproducibility and stability make Nilotinib Capsules suitable for:
In vitro assays requiring precise concentration control.
Animal studies modeling leukemia progression and response to tyrosine kinase inhibition.
Mechanistic studies dissecting BCR-ABL-mediated signaling and apoptotic pathways.
Summary
In summary, Nilotinib Capsules (CAS 641571-10-0) provide a high-purity, research-grade BCR-ABL tyrosine kinase inhibitor for preclinical leukemia studies. Their capsule format ensures precise dosing and experimental reproducibility, making them a reliable tool for:
Mechanistic studies of leukemia cell proliferation and apoptosis
Investigation of drug resistance and kinase signaling pathways
Combination therapy research and translational preclinical modeling
Product Specifications
| Parameter | Specification |
|---|---|
| Chemical Name / Synonyms | Nilotinib, AMN107, Tasigna, BCR-ABL inhibitor |
| CAS Number | 641571-10-0 |
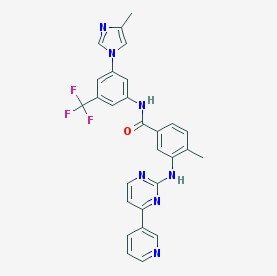
| Molecular Formula | C28H22F3N7O |
| Molecular Weight | 529.5 g/mol |
| Purity | ≥99% (HPLC) |
| Appearance | White to off-white crystalline powder in capsule form |
| Dosage Form | Capsules |
| Solubility | Slightly soluble in water, soluble in DMSO, ethanol |
| Concentration | Customizable for preclinical research; typical dose range: 1–10 mg per capsule equivalent |
| Storage Temperature | 2–8°C, protect from light and moisture |
| Stability | Stable for ≥24 months under recommended storage conditions |
| Analytical Methods | HPLC, LC-MS/MS, NMR spectroscopy, UV-Vis spectrophotometry |
| Mechanistic Target | BCR-ABL tyrosine kinase, including mutant variants |
| Structural Category | Small-molecule kinase inhibitor |
| Applications | Preclinical leukemia research, BCR-ABL signaling studies, drug resistance assays, combination therapy evaluation |
| Batch Consistency | GMP-compliant production ensuring reproducible purity, potency, and bioactivity |
| Regulatory Notes | For research use only; not intended for human or veterinary use |
| Origin | China, GMP-certified production facility |
| Additional Data | Certificates of Analysis (CoA) provided; stability and potency validated under experimental conditions; endotoxin levels <0.1 EU/mg |
Detailed Description:
Nilotinib Capsules provide a high-purity, research-grade formulation for preclinical studies of BCR-ABL-positive leukemia. The capsules allow reproducible dosing in both in vitro cell culture assays and in vivo animal models, supporting investigations into cellular proliferation, apoptosis, and kinase signaling pathways. GMP production guarantees consistent functional activity, making these capsules suitable for mechanistic studies of drug resistance, combination therapies, and translational research.
Capsule formulation reduces handling variability compared to powders and provides precise delivery of the active compound in leukemia cell lines, xenograft models, or pharmacodynamic assays. Purity verification using HPLC, LC-MS/MS, and NMR ensures experimental reproducibility and supports high-impact mechanistic research.
Mechanism of Action
Nilotinib Capsules (CAS 641571-10-0) are a highly selective BCR-ABL tyrosine kinase inhibitor, designed to target both wild-type and mutant BCR-ABL proteins, including forms resistant to first-generation inhibitors like imatinib. The BCR-ABL fusion protein, resulting from the Philadelphia chromosome translocation (t(9;22)(q34;q11)), exhibits constitutive kinase activity, driving uncontrolled proliferation and survival of leukemic cells. Nilotinib binds to the ATP-binding site of BCR-ABL, preventing substrate phosphorylation and downstream signal propagation.
1. BCR-ABL Kinase Inhibition
Upon administration in preclinical models or in vitro systems, Nilotinib Capsules bind selectively to the ATP-binding pocket of BCR-ABL, effectively inhibiting its kinase activity. This blockade prevents phosphorylation of downstream targets, including CRKL, STAT5, and MAPK/ERK, thereby halting aberrant leukemic cell signaling. Key experimental outcomes include:
Arrested cell cycle progression in the G1 phase
Induction of apoptotic pathways via caspase-3 activation
Suppression of proliferation in BCR-ABL-positive leukemic cells
High-purity Nilotinib Capsules ensure that observed effects are specific to BCR-ABL inhibition, minimizing confounding effects from impurities or batch variability.
2. Downstream Signaling Pathways
Nilotinib influences multiple intracellular pathways central to leukemia pathophysiology:
MAPK/ERK pathway: Normally promotes cell proliferation; inhibited by Nilotinib, leading to growth suppression.
PI3K/Akt pathway: Supports cell survival; blockade induces apoptosis.
STAT5 signaling: Drives transcription of pro-survival genes; inhibited upon Nilotinib treatment, reducing leukemic cell viability.
CRKL phosphorylation: Reduced CRKL activation serves as a biomarker for Nilotinib efficacy in preclinical assays.
These molecular effects are measurable using western blot, flow cytometry, phospho-specific antibodies, and reporter assays, allowing quantitative assessment of mechanistic outcomes.
3. Induction of Apoptosis
Nilotinib Capsules trigger programmed cell death through both intrinsic and extrinsic apoptotic pathways:
Mitochondrial depolarization and release of cytochrome c
Caspase-3 and -9 activation leading to DNA fragmentation
Modulation of pro- and anti-apoptotic proteins, including Bax, Bcl-2, and survivin
Preclinical studies using Nilotinib Capsules confirm dose-dependent apoptotic responses in BCR-ABL-positive leukemia cell lines, validating the drug’s mechanism and providing insights into resistance mechanisms.
4. Overcoming Drug Resistance
Nilotinib is particularly valuable in resistant leukemia models:
Inhibits BCR-ABL mutants (e.g., Y253H, E255K, F359V) resistant to first-generation inhibitors
Reduces clonal expansion of resistant leukemic cells
Provides a tool for mechanistic studies of secondary resistance and kinase domain mutations
Researchers can use Nilotinib Capsules to evaluate combination therapies and alternative dosing strategies in resistant models, supporting translational preclinical research.
5. Preclinical Applications
Nilotinib Capsules are utilized in multiple research contexts:
In vitro proliferation assays in leukemia cell lines
Apoptosis and cytotoxicity studies for mechanistic elucidation
Signal transduction analysis of MAPK/ERK, PI3K/Akt, and STAT5 pathways
Combination therapy research: synergistic effects with other kinase inhibitors, chemotherapeutics, or targeted therapies
Biomarker identification for BCR-ABL activity and response prediction
The capsules provide consistent dosing, enabling reproducible results across experimental models, including both cellular and animal studies.
6. Summary
In summary, Nilotinib Capsules (CAS 641571-10-0) act as a potent and selective BCR-ABL tyrosine kinase inhibitor, effectively halting leukemic cell proliferation, inducing apoptosis, and modulating key intracellular signaling pathways. Their high-purity, research-grade formulation makes them a reliable tool for mechanistic leukemia studies, drug resistance investigations, and combination therapy research, supporting both in vitro and in vivo preclinical models.
Side Effects
Nilotinib Capsules (CAS 641571-10-0), while designed exclusively for preclinical and laboratory research, exhibit measurable cellular and molecular effects that are critical for researchers to consider when designing experiments. These effects are consistent with its mechanism as a BCR-ABL tyrosine kinase inhibitor and are valuable for mechanistic studies but require careful experimental design to avoid confounding outcomes.
1. Cellular Effects
Exposure to Nilotinib Capsules in cell culture systems leads to:
Inhibition of proliferation: BCR-ABL-positive leukemia cells show a dose-dependent reduction in growth.
Induction of apoptosis: Observed via caspase activation, Annexin V staining, and DNA fragmentation.
Altered cell cycle dynamics: Arrest in the G1 phase due to inhibited kinase signaling.
While these effects are intended for mechanistic studies, excessive concentrations can result in non-specific cytotoxicity, affecting control cell populations.
2. Molecular and Signaling Effects
At the molecular level, Nilotinib blocks BCR-ABL-mediated phosphorylation, resulting in:
Downregulation of CRKL phosphorylation, a direct marker of BCR-ABL activity.
Inhibition of MAPK/ERK and PI3K/Akt pathways, reducing survival signaling.
Suppression of STAT5 activation, decreasing transcription of anti-apoptotic genes.
Modulation of apoptotic proteins, including Bax, Bcl-2, and survivin, leading to programmed cell death.
These effects are experimentally desirable but should be quantified carefully to distinguish between specific BCR-ABL inhibition and off-target kinase effects.
3. Laboratory Handling Considerations
Although formulated in capsules for precise dosing, Nilotinib remains a bioactive small molecule. Proper laboratory handling includes:
PPE: Gloves, lab coat, and eye protection.
Use of fume hoods for powder transfer or capsule dissolution.
Avoiding repeated freeze-thaw cycles to maintain activity.
Proper disposal of residual material to prevent environmental contamination.
Following these precautions ensures both safety and reproducibility of experimental results.
4. Off-Target Effects
High concentrations of Nilotinib Capsules may lead to off-target kinase inhibition, including c-KIT, PDGFR, and other tyrosine kinases. Researchers should:
Conduct dose-response studies to identify optimal experimental concentrations.
Include appropriate negative controls and isotype controls.
Validate findings using complementary techniques such as siRNA knockdown or CRISPR-mediated gene editing.
These steps minimize confounding results in mechanistic and translational studies.
5. Summary
In preclinical research, Nilotinib Capsules (CAS 641571-10-0) effectively inhibit BCR-ABL activity, modulate downstream signaling, induce apoptosis, and halt proliferation in leukemia models. Awareness of cellular and molecular effects, proper laboratory handling, and careful experimental design are essential to ensure reproducible results. These features make Nilotinib Capsules an indispensable tool for mechanistic leukemia research, drug resistance studies, and preclinical therapy evaluation.
Keywords
Nilotinib Capsules, CAS 641571-10-0, BCR-ABL tyrosine kinase inhibitor, chronic myeloid leukemia research, leukemia cell line study, drug resistance research, preclinical leukemia models, kinase signaling modulation, high-purity small molecule, mechanistic leukemia study, translational cancer research, combination therapy evaluation, factory small-molecule supplier, China chemical manufacturer, bulk production research-grade, high-purity research reagent.
Shipping Guarantee
Nilotinib Capsules (CAS 641571-10-0) are shipped under strict temperature-controlled conditions to preserve chemical stability and activity. Packaging is insulated, tamper-evident, and includes real-time tracking and insurance coverage. International delivery options, express shipping, and cold-chain logistics ensure that capsules arrive at research laboratories in optimal condition for preclinical and mechanistic studies.
Trade Assurance
All batches of Nilotinib Capsules are produced under GMP-compliant standards with purity ≥99% verified by HPLC, LC-MS/MS, and NMR. Certificates of Analysis (CoA) accompany each lot. Bulk production, OEM, and customized concentrations are available. The manufacturer provides reliable replacement or return policies, ensuring consistent experimental reproducibility.
Payment Support
Payment options include PayPal, major credit cards, T/T, USDT, Bitcoin, and Ethereum. Transactions are fully encrypted, enabling secure and global procurement of high-purity Nilotinib Capsules for preclinical leukemia research and mechanistic studies.
Disclaimer
Nilotinib Capsules (CAS 641571-10-0) are intended exclusively for laboratory and preclinical research use. They are not for human or veterinary administration. Researchers must follow biosafety protocols, use appropriate PPE, and handle capsules according to GLP standards. Improper use may alter experimental outcomes. All content is provided for scientific research purposes only.
References
PubChem – Nilotinib
Provides detailed molecular structure, physicochemical properties, and bioactivity data for Nilotinib, supporting preclinical leukemia research.ChEMBL – Nilotinib
Contains bioactivity profiles, molecular target interactions, and experimental assay data suitable for mechanistic studies of BCR-ABL inhibition.DrugBank – Nilotinib
Provides pharmacological information, mechanism of action insights, and preclinical applications of Nilotinib Capsules.PubMed – Nilotinib Research Articles
Access peer-reviewed publications detailing BCR-ABL inhibition, leukemia cell proliferation, apoptosis, and resistance mechanisms.IUPHAR/BPS – Tyrosine Kinase Targets
Curated data on kinase targets, signaling pathways, and molecular interactions relevant to Nilotinib Capsules research.Additional Resources
Sigma-Aldrich Technical Datasheets on Nilotinib.
ResearchGate Experimental Studies on BCR-ABL Inhibitors.
These references provide authoritative, peer-reviewed information for laboratories using Nilotinib Capsules (CAS 641571-10-0) in mechanistic, preclinical, and translational leukemia research. They support molecular data, functional assay protocols, and experimental design for reproducible and high-impact studies.

Additional information
| Weight | 1.1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
1. What is the primary use of Nilotinib Capsules?
Nilotinib Capsules (CAS 641571-10-0) are used in preclinical leukemia research, targeting BCR-ABL tyrosine kinase to study cellular proliferation, apoptosis, and kinase signaling pathways.
2. How should Nilotinib Capsules be stored?
Store at 2–8°C, protect from light and moisture, and avoid repeated freeze-thaw cycles to maintain high-purity and activity.
3. Are these capsules GMP-compliant?
Yes, all batches meet GMP standards, with purity ≥99% and validated functional activity for reproducible research results.
4. Can Nilotinib Capsules be used in combination studies?
Yes, they are compatible with chemotherapeutics, kinase inhibitors, or other targeted agents for synergistic preclinical studies.
5. Does each batch include a Certificate of Analysis?
Yes, each batch includes a CoA, confirming purity, identity, and functional activity for reliable experimental use.
6. Is bulk production or OEM available?
Yes, our factory small-molecule supplier provides bulk production and OEM services for research labs and preclinical studies.
7. Are Nilotinib Capsules suitable for in vitro assays?
Yes, they are ideal for cell proliferation, apoptosis, and kinase signaling studies in leukemia cell lines.
8. Can they be used in in vivo preclinical models?
Yes, capsules can be formulated for dosing in animal models to study leukemia progression and response to BCR-ABL inhibition.
9. Which signaling pathways are affected?
Nilotinib modulates MAPK/ERK, PI3K/Akt, STAT5, and other BCR-ABL downstream pathways, enabling mechanistic studies.
10. What are key laboratory precautions?
Use PPE, handle in a fume hood if needed, avoid contamination, and properly dispose of unused capsules to maintain safety.
11. Can they help study drug resistance?
Yes, Nilotinib inhibits BCR-ABL mutants resistant to first-generation inhibitors, supporting mechanistic drug resistance research.
12. How long is the shelf-life?
Stable for ≥24 months under recommended storage, preserving structural integrity and functional activity.
13. Are high-throughput studies possible?
Yes, Nilotinib is compatible with multi-well plate-based assays for mechanistic and combinatorial studies.
14. Who supplies high-purity Nilotinib Capsules globally?
Reputable China chemical manufacturers and factory small-molecule suppliers provide GMP-grade, high-purity research capsules for global laboratories.
15. Can Nilotinib Capsules be used for biomarker studies?
Yes, they are suitable for analyzing BCR-ABL phosphorylation, downstream kinase activity, and apoptotic markers in preclinical studies.














Reviews
There are no reviews yet.