No products in the cart.
Sale
Nivolumab | CAS 946414-94-4 | PD-1 Immune Checkpoint Inhibitor for Cancer Immunotherapy Research
Original price was: $3.00.$2.00Current price is: $2.00.
Nivolumab (CAS 946414-94-4) is a fully human IgG4 monoclonal antibody targeting the programmed death-1 (PD-1) receptor. It is widely utilized in cancer immunotherapy research for investigating T-cell activation, tumor immune evasion mechanisms, and checkpoint blockade pathways in oncology.
Description
Product Description
Nivolumab is a fully human IgG4 monoclonal antibody specifically designed to block the programmed cell death protein 1 (PD-1) receptor found on activated T-cells. This checkpoint molecule plays a central role in immune regulation by inhibiting overactivation of T-cells, thus preventing autoimmunity. However, many cancer cells exploit the PD-1 pathway to evade immune detection. Research involving Nivolumab provides critical insight into immune checkpoint blockade mechanisms and the reactivation of antitumor immunity.
The compound, identified by CAS 946414-94-4, has become a cornerstone of immuno-oncology research due to its precise targeting and reproducible biological effects. Nivolumab binds to PD-1 with high affinity, preventing its interaction with ligands PD-L1 and PD-L2, thereby restoring the immune system’s ability to recognize and destroy tumor cells.
Nivolumab is not a small molecule or peptide but a recombinant monoclonal antibody produced through mammalian cell culture technology. It belongs to the class of immune checkpoint inhibitors and is widely employed as a biological reagent in in vitro and in vivo studies focusing on tumor immunology, immune cell signaling, and adaptive resistance mechanisms.
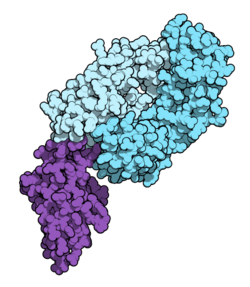
Structural and Biochemical Features
Type: Fully human IgG4 monoclonal antibody
Molecular Target: Programmed cell death protein 1 (PD-1)
Host Cell Expression System: Chinese Hamster Ovary (CHO) cells
Molecular Weight: Approximately 146 kDa
Form: Lyophilized powder or sterile liquid
Storage: 2–8°C (short-term), −20°C (long-term)
Solubility: Soluble in water-based buffers (PBS, saline)
Nivolumab’s structure includes two heavy and two light chains, forming a Y-shaped immunoglobulin. The Fab regions specifically bind PD-1, while the Fc region of IgG4 has reduced effector function, minimizing unwanted immune activation during experiments.
Research Applications
Nivolumab serves as a powerful model antibody in experimental oncology and immunology. Researchers use it to study:
Checkpoint Blockade Mechanisms:
Nivolumab blocks PD-1/PD-L1 signaling, reactivating T-cell cytotoxic functions against tumor cells. It provides a research model for immune reprogramming and T-cell exhaustion reversal.T-Cell Activation and Signaling Studies:
It is widely used to examine phosphorylation cascades, cytokine production, and transcriptional reactivation of immune effector cells.Cancer Immunotherapy Research:
Nivolumab has become essential in preclinical models of melanoma, lung cancer, renal carcinoma, and Hodgkin lymphoma to understand immune resistance.Biomarker Discovery:
Studies involving PD-1 blockade help identify predictive biomarkers for treatment response, including PD-L1 expression and tumor mutational burden (TMB).Combination Therapy Models:
In laboratory studies, Nivolumab is often combined with CTLA-4 inhibitors, kinase blockers, or radiation models to evaluate synergistic immune activation.
Molecular Characteristics and Advantages in Research
High Target Specificity: Selective inhibition of PD-1 without affecting other immune receptors.
Human-Derived Framework: Reduced immunogenicity in biological models.
Stable Glycosylation Profile: Ensures reproducibility and consistency across experimental batches.
Well-Characterized Mechanism: Extensively studied for checkpoint blockade biology, making it ideal for comparative research.
Nivolumab’s development represents a major advancement in the field of immune-oncology, inspiring generations of checkpoint inhibitors and monoclonal antibody research.
Product Specifications
| Item | Specification |
|---|---|
| Product Name | Nivolumab |
| CAS Number | 946414-94-4 |
| Molecular Formula | C6428H9964N1732O1996S42 (approximate) |
| Molecular Weight | ~146 kDa |
| Type | Human IgG4 monoclonal antibody |
| Target | PD-1 (Programmed Death-1 receptor) |
| Purity | ≥99% |
| Appearance | White to off-white lyophilized powder |
| Expression System | CHO cells |
| Form | Lyophilized or liquid |
| Solubility | Soluble in PBS and isotonic buffers |
| Storage | 2–8°C (short-term), −20°C (long-term) |
| Synonyms | MDX-1106, BMS-936558, ONO-4538 |
| Research Area | Immunology, oncology, cell signaling |
| Intended Use | For laboratory research use only |
Mechanism of Action
Nivolumab operates through immune checkpoint blockade, a transformative mechanism in immuno-oncology research. The PD-1 receptor, expressed on activated T-cells, functions as a negative regulator that dampens immune responses when engaged by its ligands PD-L1 and PD-L2. Tumor cells frequently overexpress these ligands, effectively “turning off” cytotoxic T-cells and evading immune destruction.
1. PD-1 Pathway and Immune Suppression
When PD-L1 binds to PD-1, the receptor recruits SHP-2 phosphatases, which dephosphorylate key signaling molecules in the T-cell receptor (TCR) pathway, including CD3ζ and ZAP70. This halts downstream activation of PI3K/Akt and MAPK pathways, leading to T-cell exhaustion, anergy, or apoptosis.
2. Nivolumab-Mediated PD-1 Blockade
Nivolumab binds directly to the extracellular domain of PD-1, sterically blocking its interaction with PD-L1 and PD-L2. By interrupting this inhibitory signal, Nivolumab restores phosphorylation of TCR components, reactivating cytokine production (IFN-γ, IL-2) and cytotoxic granule release (perforin, granzyme B).
3. Restoration of T-Cell Function
Through PD-1 inhibition, Nivolumab enables:
Enhanced T-cell proliferation
Increased cytotoxicity against tumor-associated antigens
Elevated interferon-gamma secretion
Improved antigen presentation via dendritic cell activation
4. Tumor Microenvironment Modulation
Research has shown that PD-1 blockade by Nivolumab alters the tumor immune microenvironment, promoting infiltration of CD8+ effector T-cells and reducing suppressive immune populations such as Tregs and myeloid-derived suppressor cells (MDSCs).
Signaling Cascade Summary
PD-1 binding → SHP-2 recruitment → TCR dephosphorylation → Inhibition
Nivolumab binding → PD-L1 blockade → TCR signaling restoration → Activation
Downstream → Cytokine release → Tumor cell lysis
This precise control of T-cell signaling makes Nivolumab an indispensable reagent for dissecting immune checkpoint mechanisms in research environments.
Side Effects
In laboratory research models, Nivolumab’s effects can induce immune-related changes reflecting enhanced immune activation:
Cytokine Release:
Elevated IFN-γ, TNF-α, and IL-2 levels observed in in vitro assays of T-cell activation.Cellular Proliferation:
Overactivation of immune effector cells, potentially leading to T-cell exhaustion in long-term culture systems.Tissue Reactivity Models:
Immune-mediated inflammation in organoid or animal models due to widespread checkpoint removal.Autoimmunity Markers:
Increased autoantibody production in autoimmune-prone model systems.Metabolic Impact:
Modulated glucose metabolism and mitochondrial activation in immune cell subsets.
These phenomena provide valuable data for mechanistic studies on immune regulation, inflammation, and autoimmunity under checkpoint blockade conditions.
Keywords
Nivolumab; CAS 946414-94-4; PD-1 inhibitor; immune checkpoint antibody; cancer immunotherapy research; monoclonal antibody reagent; oncology biomarker study; high purity biologicals; research peptide manufacturer; OEM antibody production; China antibody supplier.
Shipping Guarantee
All shipments are handled using validated cold-chain logistics to preserve protein structure and bioactivity. Each package is sealed in moisture-proof containers with secondary protective wrapping and continuous temperature monitoring. Products are shipped via express international couriers with full tracking and insurance coverage.
Trade Assurance
We ensure product authenticity, verified ≥99% purity, and compliance with analytical standards (HPLC, SDS-PAGE, and ELISA). Each batch is supplied with a Certificate of Analysis (CoA). Our trade assurance policy guarantees replacement or refund for any deviation from listed specifications.
Payment Support
We provide flexible and secure global payment options to support international research transactions. Accepted payment methods include PayPal, major credit cards (Visa, MasterCard, American Express), telegraphic transfer (T/T), and cryptocurrencies (USDT, Bitcoin, Ethereum). All transactions are protected by industry-standard encryption and verified payment gateways to ensure confidentiality and fund security.
Disclaimer
This product is intended for laboratory research use only. It is not for human or veterinary use, nor for diagnostic or therapeutic applications. Researchers must handle this antibody following institutional biosafety guidelines.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 18 × 16 × 18 cm |
Q1: What is the main function of Nivolumab in research?
A1: It inhibits PD-1 signaling, restoring T-cell activation and antitumor immune responses in experimental models.
Q2: What is the purity of Nivolumab supplied?
A2: The product is supplied with ≥99% purity, confirmed via HPLC and SDS-PAGE analysis.
Q3: Can Nivolumab be used for in vivo studies?
A3: Yes, it is suitable for in vitro and in vivo immunology and oncology research models.
Q4: What storage conditions are recommended?
A4: Store at 2–8°C for short-term use or −20°C for long-term preservation.
Q5: Is Nivolumab the same as PD-1 antibody?
A5: Yes, Nivolumab is a monoclonal antibody targeting PD-1, commonly referred to as a PD-1 inhibitor.
Q6: Is the antibody recombinant?
A6: Yes, Nivolumab is produced recombinantly in CHO cells to ensure consistency and high yield.
Q7: Do you offer CoA and analytical verification?
A7: Each batch includes a Certificate of Analysis and full analytical data (HPLC, ELISA).
Q8: Can bulk orders be customized for OEM production?
A8: Yes, we support OEM & bulk antibody production for laboratories and distributors.
Q9: Are you a factory supplier?
A9: Yes, we are a China-based biological reagent manufacturer offering high-purity antibody supply.
Q10: How do I confirm identity and functionality?
A10: Functionality is verified through binding assays with PD-1 and ligand inhibition tests.
Q11: Do you provide research support for immunology projects?
A11: Technical documentation and analytical reports can be provided upon request.
Q12: Is this product suitable for combination studies?
A12: Yes, it can be combined with other checkpoint inhibitors (CTLA-4, LAG-3) in research.
Q13: Can I purchase at wholesale price?
A13: Yes, we support antibody wholesale China and global distribution partnerships.
Q14: How do you ensure shipping stability?
A14: Shipped via validated cold-chain couriers with continuous temperature control.
Q15: What guarantees product authenticity?
A15: Each antibody batch undergoes multi-point verification, and our trade assurance guarantees replacement for any non-conformance.















Reviews
There are no reviews yet.