No products in the cart.
Octapharma Human Albumin 12.5?g – Wholesale & Retail (For Research Use Only)
$2.00
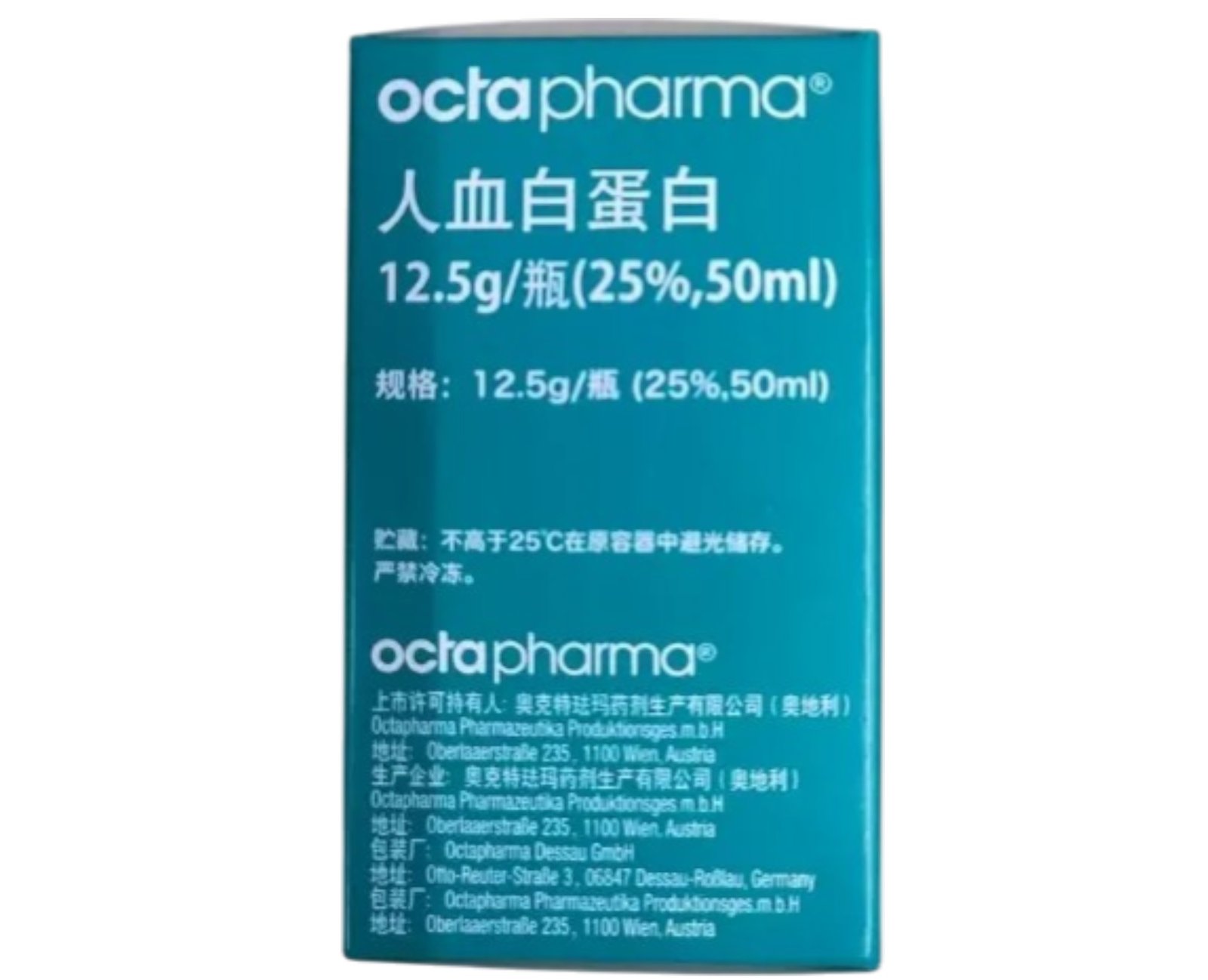
Octapharma Human Albumin 25% is a highly purified sterile albumin solution containing 12.5?g in 50?ml (25%). Manufactured by Octapharma (Austria), approved under SJ20130031. Suitable for experimental research on plasma volume expansion, protein?binding, and osmotic dynamics. Wholesale and retail available. For research use only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
Octapharma Human Albumin 25% is produced from large pools of screened human plasma and processed via cold ethanol fractionation, ultrafiltration, and pasteurization to ensure high purity and viral safety. This concentration provides potent oncotic pressure with minimal fluid load. In laboratory research, it is used to simulate hypovolemia correction, protein binding dynamics assays, colloid osmotic models, and carry?over experiments in pharmacokinetics. Packaged as 12.5?g in 50?ml single-use vials. Strictly for laboratory research use only.
Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Octapharma Human Albumin 25% |
| Strength & Volume | 12.5?g albumin in 50?ml (25%) |
| Dosage Form | Sterile liquid injection |
| Packaging Unit | 1 vial per box |
| Manufacturer | Octapharma Produktionsges.m.b.H. (Austria) |
| Approval Number | ???? SJ20130031 |
| Drug Standard Code | 86979355000136 |
| Barcode | 9006477056411 |
| CAS Number | Polyclonal human serum albumin (no CAS assigned) |
| Molecular Type | ~66.5?kDa human serum albumin |
Mechanism of Action & Research Applications
Human albumin contributes significantly to colloid osmotic pressure, stabilizes circulating volume, and binds hormones, drugs, fatty acids, and bilirubin. In research, Octapharma Human Albumin 25% is frequently used to model rapid plasma volume expansion, assess drug-binding kinetics, mimic critical care fluid therapy, and explore transport protein dynamics in cell culture, animal models, and pharmacological studies.
Side Effects (For Reference Only in Research Models)
Model systems or analogous clinical observations may include transient hypertension or hypotension, fluid overload, allergic reactions, headaches, and rare thrombotic events. These findings guide safety monitoring and dose planning in experimental designs.
Disclaimer
Octapharma Human Albumin 25% is strictly intended for laboratory research use only. Not for human or veterinary therapeutic or diagnostic use.
Additional information
| Weight | 1 kg |
|---|---|
| Dimensions | 36 × 23 × 36 cm |











Reviews
There are no reviews yet.