No products in the cart.
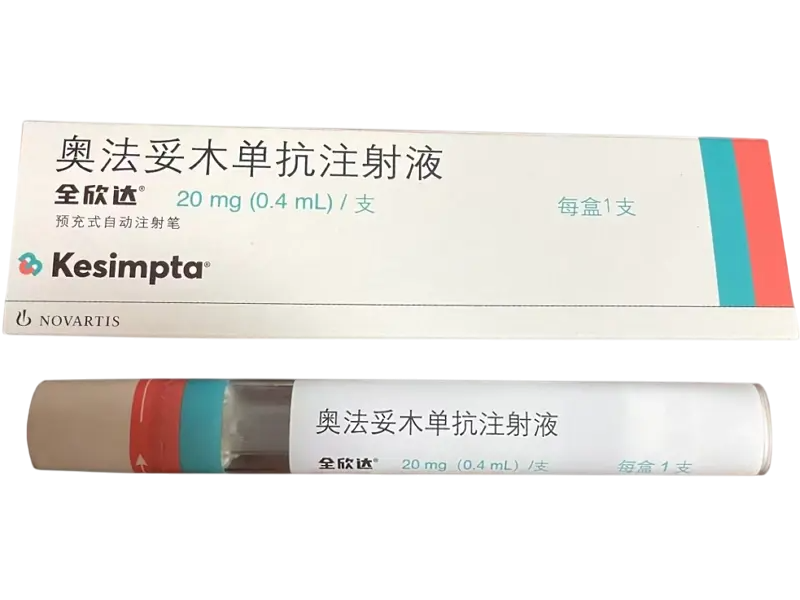
Ofatumumab (Kesimpta®) 20?mg Prefilled Autoinjector Pen – For Research Use Only
$2.00
Ofatumumab (Kesimpta®) is a fully human anti-CD20 monoclonal antibody, supplied as a 20?mg/0.4?mL prefilled auto-injection pen. Manufactured by Novartis under SJ20210034. Ideal for B?cell depletion models, neuroimmunology and autoimmune research. Wholesale & retail supported. For research use only.?For wholesale prices, other specifications and uses, please consult our staff?
Description
Ofatumumab (Kesimpta®) is a fully human IgG1k monoclonal antibody targeting the CD20 antigen on B?lymphocytes. It achieves targeted B?cell lysis and rapid depletion when administered subcutaneously. Approved for relapsing multiple sclerosis, it is widely used in research for modeling B?cell driven immune responses, multiple sclerosis pathology, CAR?T therapy B?cell reconstitution, and autoimmune disease signaling. Its autoinjector pen allows precise, monthly dosing in research protocols. For laboratory research use only.
Product Specifications
| Parameter | Detail |
|---|---|
| Product Name | Ofatumumab (Kesimpta®) Injection |
| Synonyms | Ofatumumab; anti-CD20 antibody; OMB157 |
| Strength | 20?mg per 0.4?mL prefilled pen |
| Dosage Form | Subcutaneous auto-injector |
| Packaging | 1 pen per box |
| Manufacturer | Novartis Pharma Stein AG, Switzerland |
| Approval Number | SJ20210034 |
| Drug Standard Code | 86979584000051 |
| Barcode | Not yet assigned |
| CAS Number | 679818?59?8 |
| Molecular Type | Fully human anti?CD20 IgG1 antibody (~148?kDa) |
Mechanism of Action & Research Applications
Ofatumumab binds CD20 on mature B?cells, inducing complement?mediated cytotoxicity and antibody?dependent cellular cytotoxicity. Subcutaneous monthly dosing enables modeling of B?cell depletion and recovery dynamics, neuroinflammatory processes, autoimmune disease progression, and CAR?T cell therapy B?cell reconstitution.
Side Effects (For Reference Only in Research Models)
Analogous human data report upper respiratory infections, injection-site and systemic reactions, headache, urinary tract infection, reduced immunoglobulin M levels, fatigue, and rare neutropenia. These observations inform model dosing safety and immunologic outcome monitoring.
Disclaimer
Ofatumumab (Kesimpta®) is strictly intended for laboratory research use only. Not for human or veterinary therapeutic, diagnostic, or prophylactic use.
Additional information
| Weight | 0.6 kg |
|---|---|
| Dimensions | 23 × 36 × 23 cm |










Reviews
There are no reviews yet.